Abstract
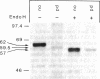
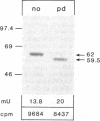
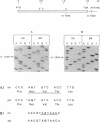
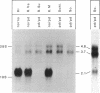
Metachromatic leukodystrophy is a metabolic disorder caused by the deficiency of arylsulfatase A. Deficiency of this enzyme is also found in apparently healthy individuals, a condition for which the term pseudodeficiency was introduced. The arylsulfatase A (cerebroside-3-sulfate 3-sulfohydrolase; EC 3.1.6.8) (ASA) encoding gene was isolated from an individual homozygous for the ASA pseudodeficiency allele. Sequence analysis revealed two A----G transitions. One changes Arg-350 to serine, which leads to the loss of a utilized N-glycosylation site. This loss explains the smaller size of ASA in ASA pseudodeficiency fibroblasts. The introduction of Ser-350 into normal ASA cDNA does not affect the rate of synthesis, the stability, or the catalytic properties of ASA in stably transfected baby hamster kidney cells. Therefore, the loss of the N-linked oligosaccharide does not contribute to the reduction of ASA activity in ASA pseudodeficiency. The other A----G transition changes the first polyadenylylation signal downstream of the stop codon from AATAAC to AGTAAC. The latter causes a severe deficiency of a 2.1-kilobase (kb) mRNA species. The deficiency of the 2.1-kb RNA species provides an explanation for the diminished synthesis of ASA seen in pseudodeficiency fibroblasts. Amplification of genomic DNA and hybridization with allele-specific oligonucleotides detected both mutations in four unrelated individuals with ASA pseudodeficiency.
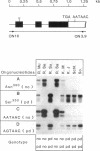
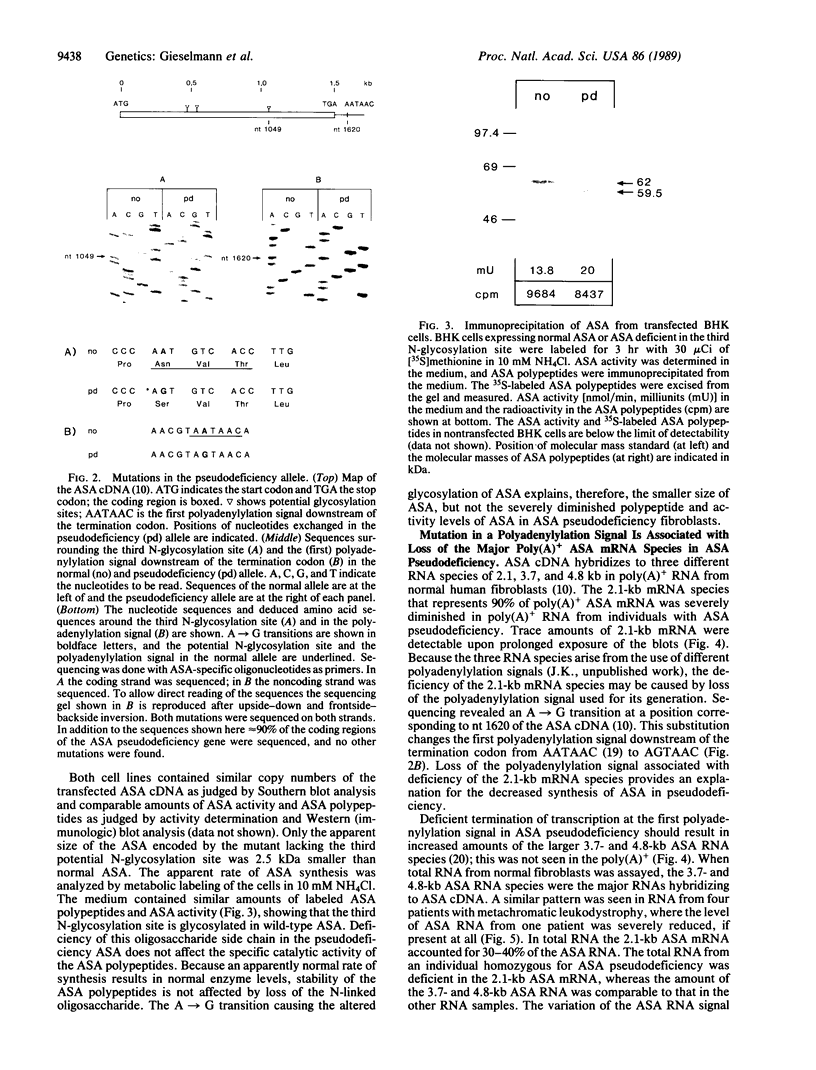
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artelt P., Morelle C., Ausmeier M., Fitzek M., Hauser H. Vectors for efficient expression in mammalian fibroblastoid, myeloid and lymphoid cells via transfection or infection. Gene. 1988 Sep 7;68(2):213–219. doi: 10.1016/0378-1119(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Baldinger S., Pierpont M. E., Wenger D. A. Pseudodeficiency of arylsulfatase A: a counseling dilemma. Clin Genet. 1987 Feb;31(2):70–76. doi: 10.1111/j.1399-0004.1987.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Burke B., Matlin K., Bause E., Legler G., Peyrieras N., Ploegh H. Inhibition of N-linked oligosaccharide trimming does not interfere with surface expression of certain integral membrane proteins. EMBO J. 1984 Mar;3(3):551–556. doi: 10.1002/j.1460-2075.1984.tb01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. L., Davidson R. G. Pseudo arylsulfatase-A deficiency in healthy individuals: genetic and biochemical relationship to metachromatic leukodystrophy. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7323–7327. doi: 10.1073/pnas.80.23.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly S., Manley J. L. A functional mRNA polyadenylation signal is required for transcription termination by RNA polymerase II. Genes Dev. 1988 Apr;2(4):440–452. doi: 10.1101/gad.2.4.440. [DOI] [PubMed] [Google Scholar]
- Conzelmann E., Sandhoff K. Partial enzyme deficiencies: residual activities and the development of neurological disorders. Dev Neurosci. 1983;6(1):58–71. doi: 10.1159/000112332. [DOI] [PubMed] [Google Scholar]
- Dubois G., Turpin J. C., Baumann N. Letter: Absence of ASA activity in healthy father of a patient with metachromatic leukodystrophy. N Engl J Med. 1975 Aug 7;293(6):302–302. doi: 10.1056/nejm197508072930613. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fluharty A. L., Meek W. E., Kihara H. Pseudo arylsulfatase A deficiency: evidence for a structurally altered enzyme. Biochem Biophys Res Commun. 1983 Apr 15;112(1):191–197. doi: 10.1016/0006-291x(83)91815-6. [DOI] [PubMed] [Google Scholar]
- Gustavson K. H., Hagberg B. The incidence and genetics of metachromatic leucodystrophy in northern Sweden. Acta Paediatr Scand. 1971 Sep;60(5):585–590. doi: 10.1111/j.1651-2227.1971.tb06994.x. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Herz B., Bach G. Arylsulfatase A in pseudodeficiency. Hum Genet. 1984;66(2-3):147–150. doi: 10.1007/BF00286589. [DOI] [PubMed] [Google Scholar]
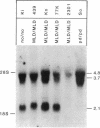
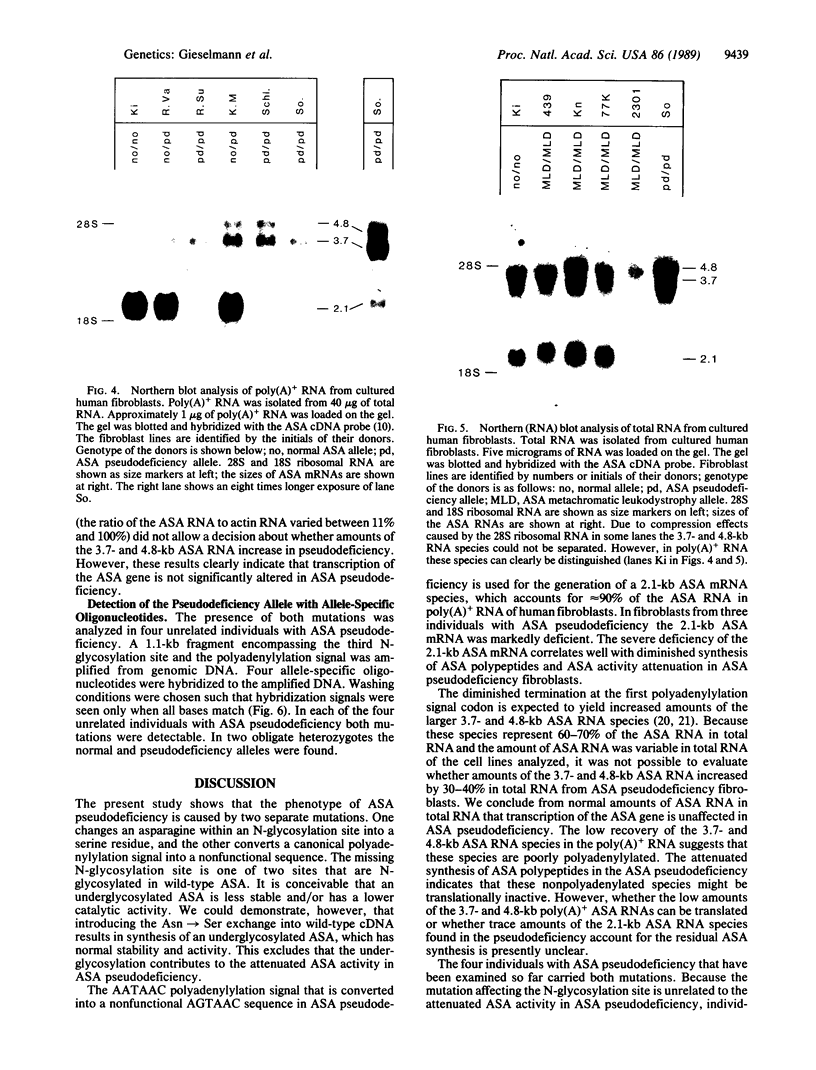
- Hohenschutz C., Eich P., Friedl W., Waheed A., Conzelmann E., Propping P. Pseudodeficiency of arylsulfatase A: a common genetic polymorphism with possible disease implications. Hum Genet. 1989 Apr;82(1):45–48. doi: 10.1007/BF00288270. [DOI] [PubMed] [Google Scholar]
- Hohenschutz C., Friedl W., Schlör K. H., Waheed A., Conzelmann E., Sandhoff K., Propping P. Probable metachromatic leukodystrophy/pseudodeficiency compound heterozygote at the arylsulfatase A locus with neurological and psychiatric symptomatology. Am J Med Genet. 1988 Sep;31(1):169–175. doi: 10.1002/ajmg.1320310120. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls R. D., Hill A. V., Clegg J. B., Higgs D. R. Direct cloning of specific genomic DNA sequences in plasmid libraries following fragment enrichment. Nucleic Acids Res. 1985 Nov 11;13(21):7569–7578. doi: 10.1093/nar/13.21.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C., Gieselmann V., Kreysing J., Schmidt B., Pohlmann R., Waheed A., Meyer H. E., O'Brien J. S., von Figura K. Cloning and expression of human arylsulfatase A. J Biol Chem. 1989 Jan 15;264(2):1252–1259. [PubMed] [Google Scholar]
- Vara J. A., Portela A., Ortín J., Jiménez A. Expression in mammalian cells of a gene from Streptomyces alboniger conferring puromycin resistance. Nucleic Acids Res. 1986 Jun 11;14(11):4617–4624. doi: 10.1093/nar/14.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Gottschalk S., Hille A., Krentler C., Pohlmann R., Braulke T., Hauser H., Geuze H., von Figura K. Human lysosomal acid phosphatase is transported as a transmembrane protein to lysosomes in transfected baby hamster kidney cells. EMBO J. 1988 Aug;7(8):2351–2358. doi: 10.1002/j.1460-2075.1988.tb03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw E., Proudfoot N. Alpha-thalassaemia caused by a poly(A) site mutation reveals that transcriptional termination is linked to 3' end processing in the human alpha 2 globin gene. EMBO J. 1986 Nov;5(11):2915–2922. doi: 10.1002/j.1460-2075.1986.tb04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Steckel F., Hasilik A. Juvenile and adult metachromatic leukodystrophy: partial restoration of arylsulfatase A (cerebroside sulfatase) activity by inhibitors of thiol proteinases. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6066–6070. doi: 10.1073/pnas.80.19.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]