Abstract
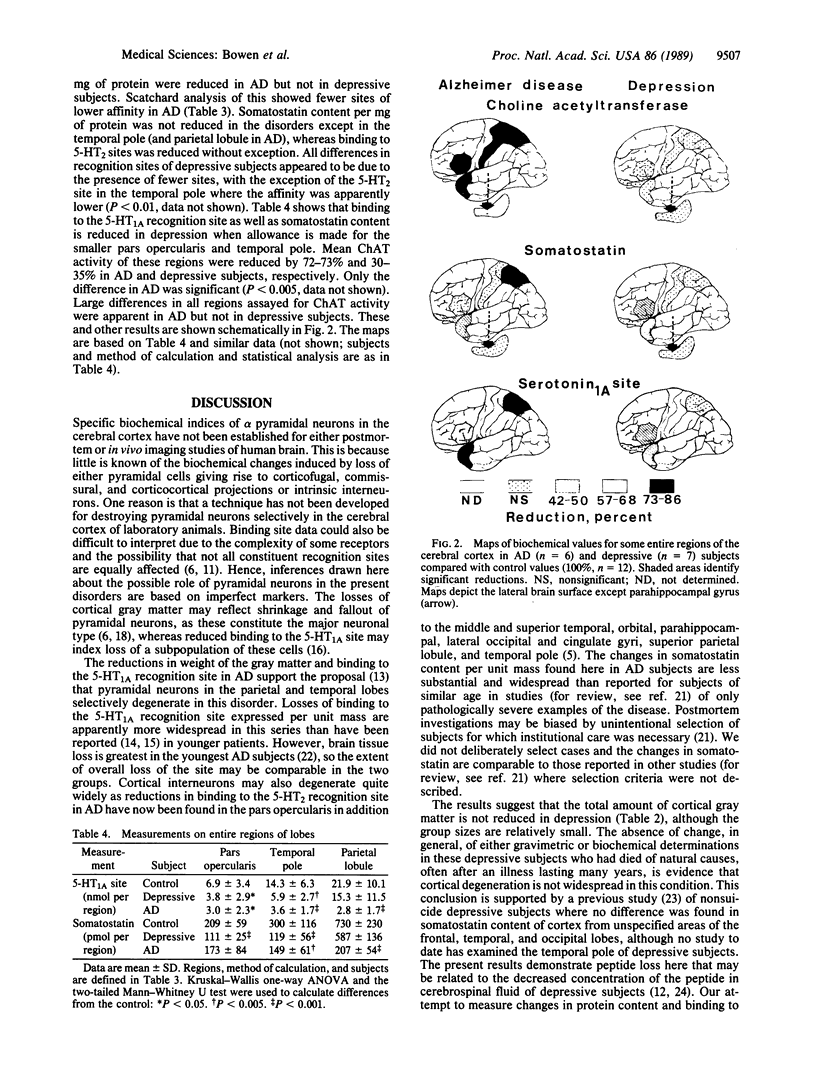
The extent and distribution of biochemical abnormalities thought to reflect disorders of subpopulations of neurons have been determined in the cerebral cortex from brains of patients with Alzheimer-type dementia and depressive illness who died of natural causes. In dementia, loss of gray matter from areas of the parietal and temporal lobes is most obvious. In depression, these areas are not affected, but the pars opercularis and temporal pole are smaller than in controls. Results expressed per unit mass of total protein indicate selective reductions in both disorders of serotonin 2 recognition sites in all areas examined and of somatostatin content in only the temporal pole of the six areas examined. In dementia alone a selective loss was found of somatostatin content of the superior parietal lobule and of serotonin 1A sites and choline acetyltransferase activity in all areas examined. Results for depression expressed per entire area indicate additionally reduced somatostatin content and serotonin 1A sites in the pars opercularis and serotonin 1A sites in the temporal pole. These multiple analyses performed on each sample provide further support for a prominent disorder of pyramidal neurons in dementia as well as more evidence for alterations in cortical neurons in depression, either as a result of the disease itself or its treatment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charlton B. G., Leake A., Wright C., Fairbairn A. F., McKeith I. G., Candy J. M., Ferrier I. N. Somatostatin content and receptors in the cerebral cortex of depressed and control subjects. J Neurol Neurosurg Psychiatry. 1988 May;51(5):719–721. doi: 10.1136/jnnp.51.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton B. G., Wright C., Leake A., Ferrier I. N., Cheetham S. C., Horton R. W., Crompton M. R., Katona C. L. Somatostatin immunoreactivity in postmortem brain from depressed suicides. Arch Gen Psychiatry. 1988 Jun;45(6):597–598. doi: 10.1001/archpsyc.1988.01800300095015. [DOI] [PubMed] [Google Scholar]
- Cheetham S. C., Crompton M. R., Katona C. L., Horton R. W. Brain 5-HT2 receptor binding sites in depressed suicide victims. Brain Res. 1988 Mar 8;443(1-2):272–280. doi: 10.1016/0006-8993(88)91621-6. [DOI] [PubMed] [Google Scholar]
- Cheetham S. C., Crompton M. R., Katona C. L., Parker S. J., Horton R. W. Brain GABAA/benzodiazepine binding sites and glutamic acid decarboxylase activity in depressed suicide victims. Brain Res. 1988 Sep 13;460(1):114–123. doi: 10.1016/0006-8993(88)91211-5. [DOI] [PubMed] [Google Scholar]
- Collerton D. Cholinergic function and intellectual decline in Alzheimer's disease. Neuroscience. 1986 Sep;19(1):1–28. doi: 10.1016/0306-4522(86)90002-3. [DOI] [PubMed] [Google Scholar]
- Davies M. F., Deisz R. A., Prince D. A., Peroutka S. J. Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Res. 1987 Oct 13;423(1-2):347–352. doi: 10.1016/0006-8993(87)90861-4. [DOI] [PubMed] [Google Scholar]
- Francis P. T., Poynton A., Lowe S. L., Najlerahim A., Bridges P. K., Bartlett J. R., Procter A. W., Bruton C. J., Bowen D. M. Brain amino acid concentrations and Ca2+-dependent release in intractable depression assessed antemortem. Brain Res. 1989 Aug 14;494(2):315–324. doi: 10.1016/0006-8993(89)90600-8. [DOI] [PubMed] [Google Scholar]
- Gold P. W., Goodwin F. K., Chrousos G. P. Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (2) N Engl J Med. 1988 Aug 18;319(7):413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- Greenamyre J. T., Young A. B. Excitatory amino acids and Alzheimer's disease. Neurobiol Aging. 1989 Sep-Oct;10(5):593–602. doi: 10.1016/0197-4580(89)90143-7. [DOI] [PubMed] [Google Scholar]
- Hubbard B. M., Anderson J. M. A quantitative study of cerebral atrophy in old age and senile dementia. J Neurol Sci. 1981 Apr;50(1):135–145. doi: 10.1016/0022-510x(81)90048-4. [DOI] [PubMed] [Google Scholar]
- Jeste D. V., Lohr J. B., Goodwin F. K. Neuroanatomical studies of major affective disorders. A review and suggestions for further research. Br J Psychiatry. 1988 Oct;153:444–459. doi: 10.1192/bjp.153.4.444. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Powell T. P. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93(4):793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Leonard B. E. Biochemical aspects of therapy-resistant depression. Br J Psychiatry. 1988 Apr;152:453–459. doi: 10.1192/bjp.152.4.453. [DOI] [PubMed] [Google Scholar]
- Lowe S. L., Francis P. T., Procter A. W., Palmer A. M., Davison A. N., Bowen D. M. Gamma-aminobutyric acid concentration in brain tissue at two stages of Alzheimer's disease. Brain. 1988 Aug;111(Pt 4):785–799. doi: 10.1093/brain/111.4.785. [DOI] [PubMed] [Google Scholar]
- McKeith I. G., Marshall E. F., Ferrier I. N., Armstrong M. M., Kennedy W. N., Perry R. H., Perry E. K., Eccleston D. 5-HT receptor binding in post-mortem brain from patients with affective disorder. J Affect Disord. 1987 Jul-Aug;13(1):67–74. doi: 10.1016/0165-0327(87)90075-9. [DOI] [PubMed] [Google Scholar]
- Middlemiss D. N., Palmer A. M., Edel N., Bowen D. M. Binding of the novel serotonin agonist 8-hydroxy-2-(di-n-propylamino) tetralin in normal and Alzheimer brain. J Neurochem. 1986 Mar;46(3):993–996. doi: 10.1111/j.1471-4159.1986.tb13069.x. [DOI] [PubMed] [Google Scholar]
- Murphy E. The prognosis of depression in old age. Br J Psychiatry. 1983 Feb;142:111–119. doi: 10.1192/bjp.142.2.111. [DOI] [PubMed] [Google Scholar]
- Najlerahim A., Bowen D. M. Regional weight loss of the cerebral cortex and some subcortical nuclei in senile dementia of the Alzheimer type. Acta Neuropathol. 1988;75(5):509–512. doi: 10.1007/BF00687139. [DOI] [PubMed] [Google Scholar]
- Palmer A. M., Lowe S. L., Francis P. T., Bowen D. M. Are post-mortem biochemical studies of human brain worthwhile? Biochem Soc Trans. 1988 Aug;16(4):472–475. doi: 10.1042/bst0160472. [DOI] [PubMed] [Google Scholar]
- Pearson R. C., Esiri M. M., Hiorns R. W., Wilcock G. K., Powell T. P. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procter A. W., Lowe S. L., Palmer A. M., Francis P. T., Esiri M. M., Stratmann G. C., Najlerahim A., Patel A. J., Hunt A., Bowen D. M. Topographical distribution of neurochemical changes in Alzheimer's disease. J Neurol Sci. 1988 Apr;84(2-3):125–140. doi: 10.1016/0022-510x(88)90118-9. [DOI] [PubMed] [Google Scholar]
- Procter A. W., Wong E. H., Stratmann G. C., Lowe S. L., Bowen D. M. Reduced glycine stimulation of [3H]MK-801 binding in Alzheimer's disease. J Neurochem. 1989 Sep;53(3):698–704. doi: 10.1111/j.1471-4159.1989.tb11760.x. [DOI] [PubMed] [Google Scholar]
- Rubinow D. R. Cerebrospinal fluid somatostatin and psychiatric illness. Biol Psychiatry. 1986 Apr;21(4):341–365. doi: 10.1016/0006-3223(86)90163-0. [DOI] [PubMed] [Google Scholar]
- Sprouse J. S., Aghajanian G. K. Responses of hippocampal pyramidal cells to putative serotonin 5-HT1A and 5-HT1B agonists: a comparative study with dorsal raphe neurons. Neuropharmacology. 1988 Jul;27(7):707–715. doi: 10.1016/0028-3908(88)90079-2. [DOI] [PubMed] [Google Scholar]
- Wilkins R. H., Brody I. A. Alzheimer's disease. Arch Neurol. 1969 Jul;21(1):109–110. doi: 10.1001/archneur.1969.00480130123013. [DOI] [PubMed] [Google Scholar]