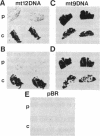
Abstract
Using in situ hybridization and immunocytochemistry, we studied a muscle biopsy sample from a patient with Kearns-Sayre syndrome (KSS) who had a deletion of mitochondrial DNA (mtDNA) and partial deficiency of cytochrome-c oxidase (COX; EC 1.9.3.1). We sought a relationship between COX deficiency and abnormalities of mtDNA at the single-fiber level. COX deficiency clearly correlated with a decrease of normal mtDNA and, conversely, deleted mtDNA was more abundant in COX-deficient fibers, especially ragged-red fibers. The distribution of mtRNA had a similar pattern, suggesting that deleted mtDNA is transcribed. Immunocytochemistry showed that the nuclear DNA-encoded subunit IV of COX was present but that the mtDNA-encoded subunit II was markedly diminished in COX-deficient ragged-red fibers. Because the mtDNA deletion in this patient did not comprise the gene encoding COX subunit II, COX deficiency may have resulted from lack of translation of mtRNA encoding all three mtDNA-encoded subunits of COX.
Full text
PDF
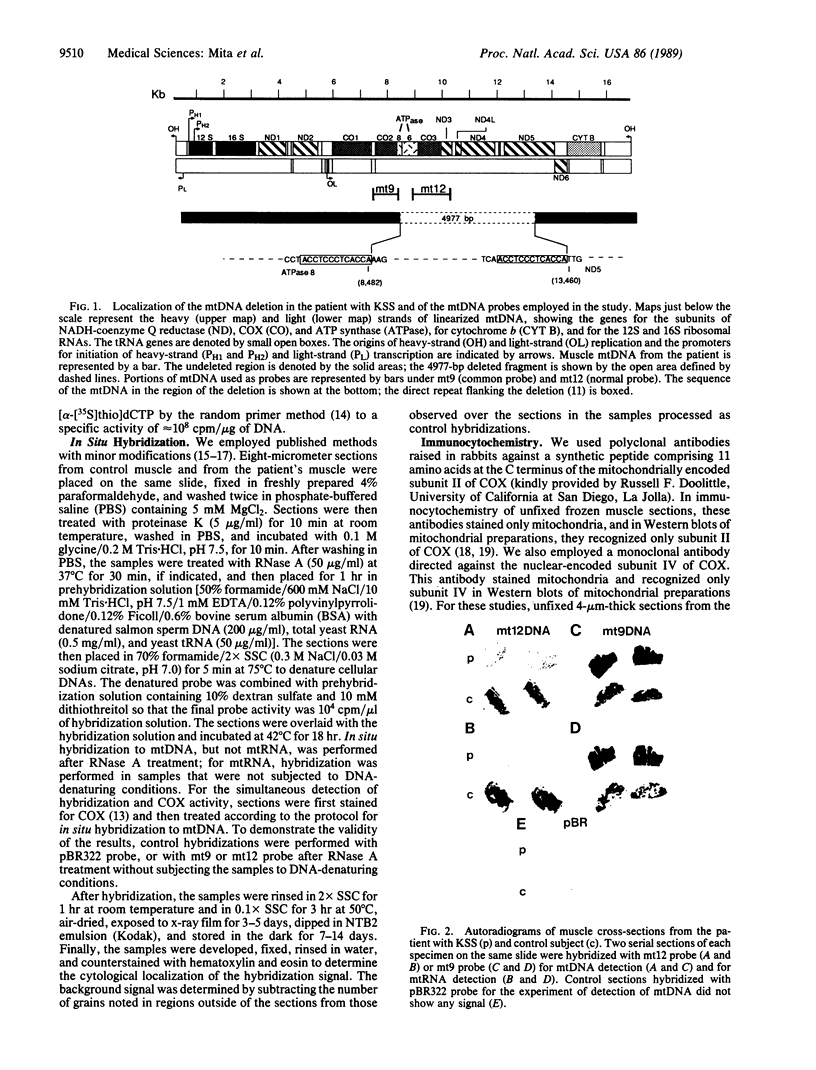
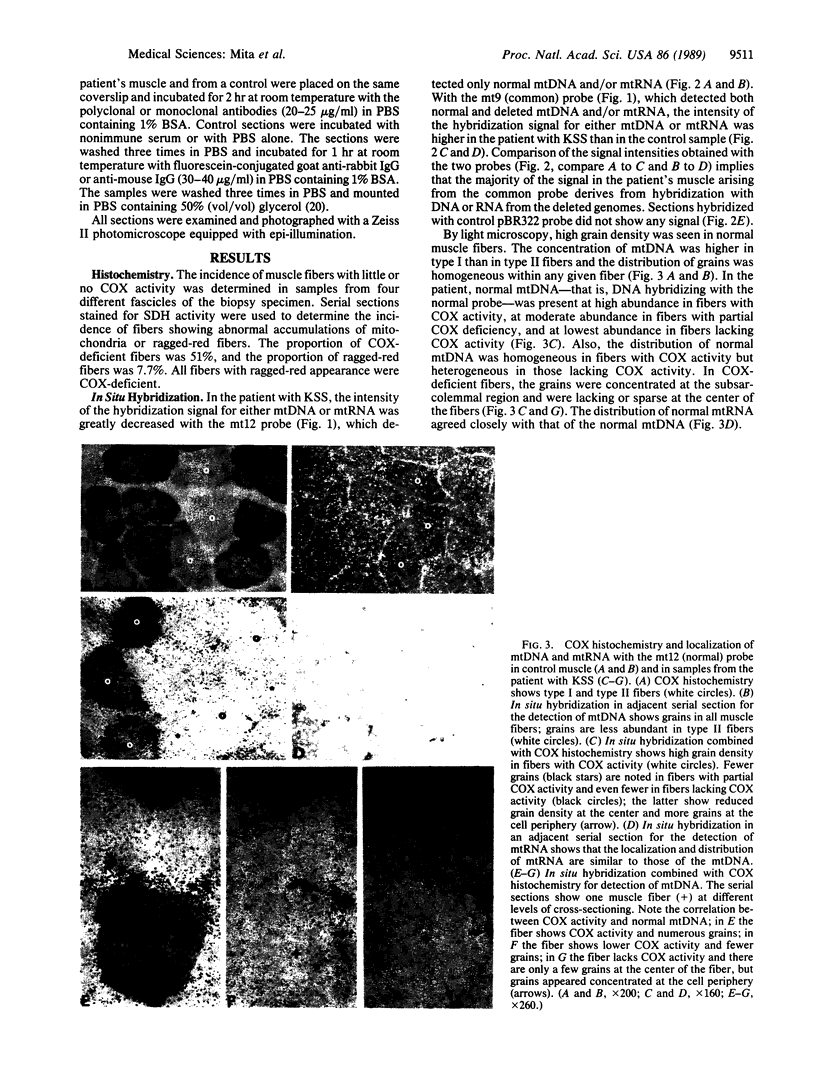


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Berenberg R. A., Pellock J. M., DiMauro S., Schotland D. L., Bonilla E., Eastwood A., Hays A., Vicale C. T., Behrens M., Chutorian A. Lumping or splitting? "Ophthalmoplegia-plus" or Kearns-Sayre syndrome? Ann Neurol. 1977 Jan;1(1):37–54. doi: 10.1002/ana.410010104. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Bonilla E., Miranda A. F., Prelle A., Salviati G., Betto R., Zeviani M., Schon E. A., DiMauro S., Rowland L. P. Immunocytochemical study of nebulin in Duchenne muscular dystrophy. Neurology. 1988 Oct;38(10):1600–1603. doi: 10.1212/wnl.38.10.1600. [DOI] [PubMed] [Google Scholar]
- Bonilla E., Schotland D. L., DiMauro S., Aldover B. Electron cytochemistry of crystalline inclusions in human skeletal muscle mitochondria. J Ultrastruct Res. 1975 Jun;51(3):404–408. doi: 10.1016/s0022-5320(75)80103-1. [DOI] [PubMed] [Google Scholar]
- Bresolin N., Moggio M., Bet L., Gallanti A., Prelle A., Nobile-Orazio E., Adobbati L., Ferrante C., Pellegrini G., Scarlato G. Progressive cytochrome c oxidase deficiency in a case of Kearns-Sayre syndrome: morphological, immunological, and biochemical studies in muscle biopsies and autopsy tissues. Ann Neurol. 1987 Jun;21(6):564–572. doi: 10.1002/ana.410210607. [DOI] [PubMed] [Google Scholar]
- Byrne E., Dennett X., Trounce I., Henderson R. Partial cytochrome oxidase (aa3) deficiency in chronic progressive external ophthalmoplegia. Histochemical and biochemical studies. J Neurol Sci. 1985 Dec;71(2-3):257–271. doi: 10.1016/0022-510x(85)90064-4. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Transcription of the mammalian mitochondrial genome. Annu Rev Biochem. 1984;53:573–594. doi: 10.1146/annurev.bi.53.070184.003041. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K. A generalized disorder of nervous system, skeletal muscle and heart resembling Refsum's disease and Hurler's syndrome. II. Ultrastructure. Am J Med. 1967 Feb;42(2):169–178. doi: 10.1016/0002-9343(67)90016-2. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Ullrich A., Saunders G. F. Localization of the human insulin gene to the distal end of the short arm of chromosome 11. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4458–4460. doi: 10.1073/pnas.78.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Turnbull D. M., Dick D. J., Sherratt H. S. A partial deficiency of cytochrome c oxidase in chronic progressive external ophthalmoplegia. J Neurol Sci. 1983 Jul;60(1):31–53. doi: 10.1016/0022-510x(83)90125-9. [DOI] [PubMed] [Google Scholar]
- Lombes A., Mendell J. R., Nakase H., Barohn R. J., Bonilla E., Zeviani M., Yates A. J., Omerza J., Gales T. L., Nakahara K. Myoclonic epilepsy and ragged-red fibers with cytochrome oxidase deficiency: neuropathology, biochemistry, and molecular genetics. Ann Neurol. 1989 Jul;26(1):20–33. doi: 10.1002/ana.410260104. [DOI] [PubMed] [Google Scholar]
- Mariottini P., Chomyn A., Doolittle R. F., Attardi G. Antibodies against the COOH-terminal undecapeptide of subunit II, but not those against the NH2-terminal decapeptide, immunoprecipitate the whole human cytochrome c oxidase complex. J Biol Chem. 1986 Mar 5;261(7):3355–3362. [PubMed] [Google Scholar]
- Mita S., Schon E. A., Herbert J. Widespread expression of amyloid beta-protein precursor gene in rat brain. Am J Pathol. 1989 Jun;134(6):1253–1261. [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., Schon E. A., DiMauro S., Miranda A. F. Heteroplasmy of mitochondrial genomes in clonal cultures from patients with Kearns-Sayre syndrome. Biochem Biophys Res Commun. 1989 Apr 28;160(2):765–771. doi: 10.1016/0006-291x(89)92499-6. [DOI] [PubMed] [Google Scholar]
- Murphy W. I., Attardi B., Tu C., Attardi G. Evidence for complete symmetrical transcription in vivo of mitochondrial DNA in HeLa cells. J Mol Biol. 1975 Dec 25;99(4):809–814. doi: 10.1016/s0022-2836(75)80187-2. [DOI] [PubMed] [Google Scholar]
- Müller-Höcker J., Pongratz D., Hübner G. Focal deficiency of cytochrome-c-oxidase in skeletal muscle of patients with progressive external ophthalmoplegia. Cytochemical-fine-structural study. Virchows Arch A Pathol Anat Histopathol. 1983;402(1):61–71. doi: 10.1007/BF00695049. [DOI] [PubMed] [Google Scholar]
- Ojala D., Merkel C., Gelfand R., Attardi G. The tRNA genes punctuate the reading of genetic information in human mitochondrial DNA. Cell. 1980 Nov;22(2 Pt 2):393–403. doi: 10.1016/0092-8674(80)90350-5. [DOI] [PubMed] [Google Scholar]
- Schon E. A., Rizzuto R., Moraes C. T., Nakase H., Zeviani M., DiMauro S. A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science. 1989 Apr 21;244(4902):346–349. doi: 10.1126/science.2711184. [DOI] [PubMed] [Google Scholar]
- Seligman A. M., Karnovsky M. J., Wasserkrug H. L., Hanker J. S. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J Cell Biol. 1968 Jul;38(1):1–14. doi: 10.1083/jcb.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy G. M., Silberberg D. H., Appel S. H., Mishkin M. M., Godfrey E. H. A generalized disorder of nervous system, skeletal muscle and heart resembling Refsum's disease and Hurler's syndrome. I. Clinical, pathologic and biochemical characteristics. Am J Med. 1967 Feb;42(2):163–168. doi: 10.1016/0002-9343(67)90015-0. [DOI] [PubMed] [Google Scholar]
- Turnbull D. M., Johnson M. A., Dick D. J., Cartlidge N. E., Sherratt H. S. Partial cytochrome oxidase deficiency without subsarcolemmal accumulation of mitochondria in chronic progressive external ophthalmoplegia. J Neurol Sci. 1985 Aug;70(1):93–100. doi: 10.1016/0022-510x(85)90191-1. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Myers A. M. Genetics of mitochondrial biogenesis. Annu Rev Biochem. 1986;55:249–285. doi: 10.1146/annurev.bi.55.070186.001341. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Maternal genes: mitochondrial diseases. Birth Defects Orig Artic Ser. 1987;23(3):137–190. [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial DNA mutations and neuromuscular disease. Trends Genet. 1989 Jan;5(1):9–13. doi: 10.1016/0168-9525(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Moraes C. T., DiMauro S., Nakase H., Bonilla E., Schon E. A., Rowland L. P. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988 Sep;38(9):1339–1346. doi: 10.1212/wnl.38.9.1339. [DOI] [PubMed] [Google Scholar]