Abstract
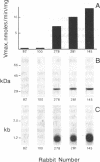
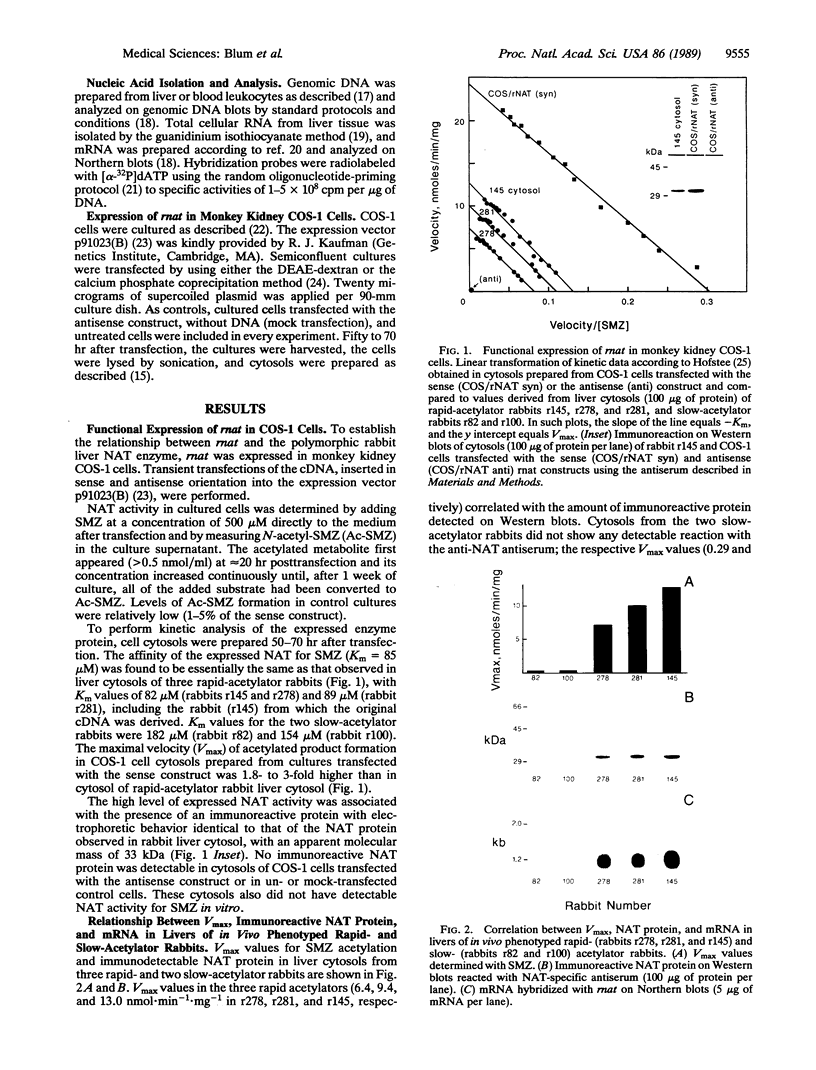
The New Zealand White rabbit provides a widely used animal model for the human acetylation polymorphism, which confers marked interindividual variation in the effect and toxicity of numerous drugs, chemicals, and potential carcinogens. The relationship of a recently isolated cDNA clone, designated rnat, to genetically polymorphic arylamine N-acetyltransferase (NAT; acetyl-CoA:arylamine N-acetyltransferase, EC 2.3.1.5) of rabbit liver was established by its expression in monkey kidney COS-1 cells: (i) cytosols from transfected cultures contained high levels of an Ac-CoA-dependent NAT activity, which was kinetically indistinguishable from that observed in cytosols from livers of genetically rapid-acetylator rabbits; (ii) transfected cells also contained an immunoreactive protein, recognized by NAT-specific antibodies, with identical electrophoretic mobility to NAT from rabbit liver. The rnat clone and anti-NAT antibodies were then used to study the relationship between NAT activity, liver enzyme protein, and the level of mRNA in livers from in vivo phenotyped rapid- and slow-acetylator rabbits. Livers from slow acetylators were devoid of both immunodetectable NAT protein and its corresponding mRNA. Analysis of genomic DNA with a panel of restriction enzymes revealed the loss of specific hybridizing bands in the DNA of slow-acetylator rabbits. These data strongly suggest that defective arylamine N-acetylation in the rabbit model is caused by a gene deletion resulting in an absence of specific mRNA and NAT enzyme protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres H. H., Vogel R. S., Tarr G. E., Johnson L., Weber W. W. Purification, physicochemical, and kinetic properties of liver acetyl-CoA:arylamine N-acetyltransferase from rapid acetylator rabbits. Mol Pharmacol. 1987 Apr;31(4):446–456. [PubMed] [Google Scholar]
- Andres H. H., Weber W. W. N-acetylation pharmacogenetics. Michaelis-Menten constants for arylamine drugs as predictors of their N-acetylation rates in vivo. Drug Metab Dispos. 1986 Jul-Aug;14(4):382–385. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M., Grant D. M., Demierre A., Meyer U. A. Nucleotide sequence of a full-length cDNA for arylamine N-acetyltransferase from rabbit liver. Nucleic Acids Res. 1989 May 11;17(9):3589–3589. doi: 10.1093/nar/17.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- EVANS D. A., MANLEY K. A., McKUSICK V. A. Genetic control of isoniazid metabolism in man. Br Med J. 1960 Aug 13;2(5197):485–491. doi: 10.1136/bmj.2.5197.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard G. A., Gammon P. T., Helmy H. S., Rees R. J. Dapsone acetylation and the treatment of leprosy. Nature. 1972 Sep 15;239(5368):159–160. doi: 10.1038/239159a0. [DOI] [PubMed] [Google Scholar]
- Evans D. A. Survey of the human acetylator polymorphism in spontaneous disorders. J Med Genet. 1984 Aug;21(4):243–253. doi: 10.1136/jmg.21.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRYMOYER J. W., JACOX R. F. INVESTIGATION OF THE GENETIC CONTROL OF SULFADIAZINE AND ISONIAZID METABOLISM IN THE RABBIT. J Lab Clin Med. 1963 Dec;62:891–904. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Blum M., Demierre A., Meyer U. A. Nucleotide sequence of an intronless gene for a human arylamine N-acetyltransferase related to polymorphic drug acetylation. Nucleic Acids Res. 1989 May 25;17(10):3978–3978. doi: 10.1093/nar/17.10.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. M., Lottspeich F., Meyer U. A. Evidence for two closely related isozymes of arylamine N-acetyltransferase in human liver. FEBS Lett. 1989 Feb 13;244(1):203–207. doi: 10.1016/0014-5793(89)81193-7. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Weber W. W. Multiple N-acetyltransferases and drug metabolism. Tissue distribution, characterization and significance of mammalian N-acetyltransferase. Biochem J. 1973 Mar;132(3):519–526. doi: 10.1042/bj1320519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein D. W., Smolen T. N., Fox R. R., Weber W. W. Identification of genetically homozygous rapid and slow acetylators of drugs and environmental carcinogens among established inbred rabbit strains. J Pharmacol Exp Ther. 1982 Oct;223(1):40–44. [PubMed] [Google Scholar]
- Herrmann B. G., Frischauf A. M. Isolation of genomic DNA. Methods Enzymol. 1987;152:180–183. doi: 10.1016/0076-6879(87)52018-3. [DOI] [PubMed] [Google Scholar]
- Küpfer A., Preisig R. Inherited defects of hepatic drug metabolism. Semin Liver Dis. 1983 Nov;3(4):341–354. doi: 10.1055/s-2008-1040786. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattila M. J., Tiitinen H. The rate of isoniazid inactivation in Finnish diabetic and non-diabetic patients. Ann Med Exp Biol Fenn. 1967;45(4):423–427. [PubMed] [Google Scholar]
- Ohsako S., Ohtomi M., Sakamoto Y., Uyemura K., Deguchi T. Arylamine N-acetyltransferase from chicken liver II. Cloning of cDNA and expression in Chinese hamster ovary cells. J Biol Chem. 1988 Jun 5;263(16):7534–7538. [PubMed] [Google Scholar]
- Patterson E., Radtke H. E., Weber W. W. Immunochemical studies of rabbit N-acetyltransferases. Mol Pharmacol. 1980 May;17(3):367–373. [PubMed] [Google Scholar]
- Platzer R., Küpfer A., Bircher J., Preisig R. Polymorphic acetylation and aminopyrine demethylation in Gilbert's syndrome. Eur J Clin Invest. 1978 Aug;8(4):219–223. doi: 10.1111/j.1365-2362.1978.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Weber W. W., Hein D. W. N-acetylation pharmacogenetics. Pharmacol Rev. 1985 Mar;37(1):25–79. [PubMed] [Google Scholar]
- Weber W. W. The molecular basis of hereditary acetylation polymorphisms. Drug Metab Dispos. 1986 Jul-Aug;14(4):377–381. [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]