Abstract
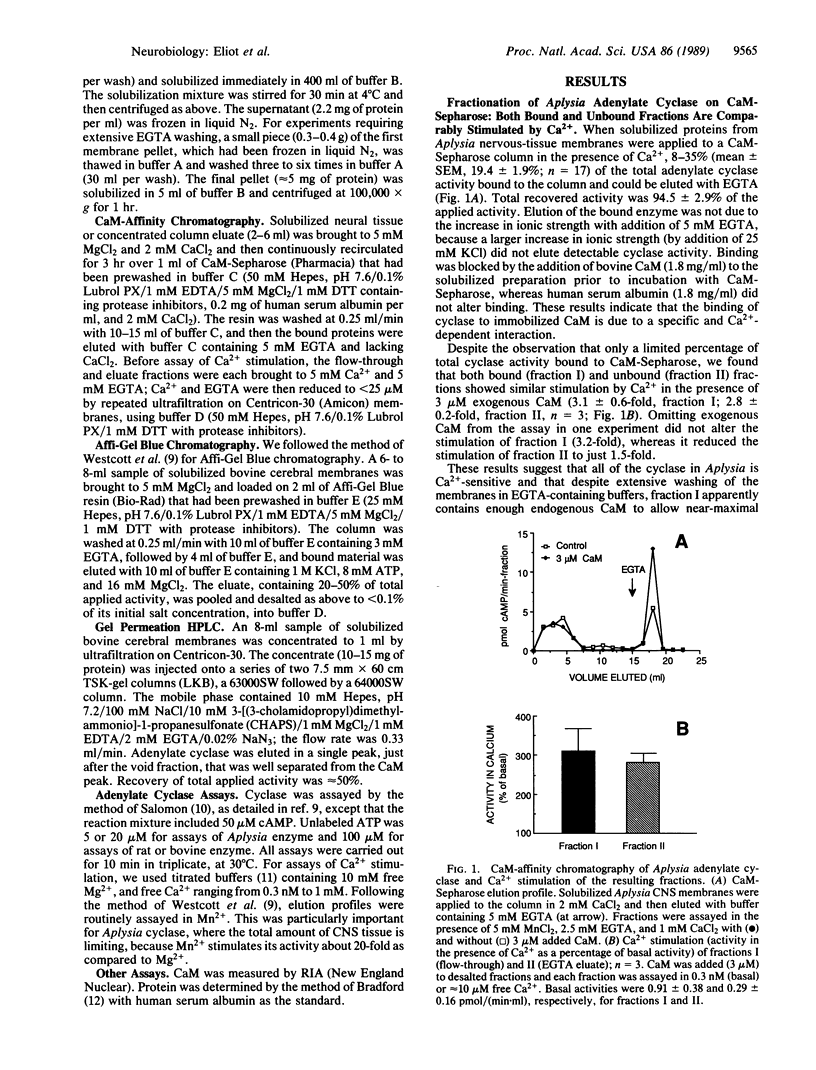
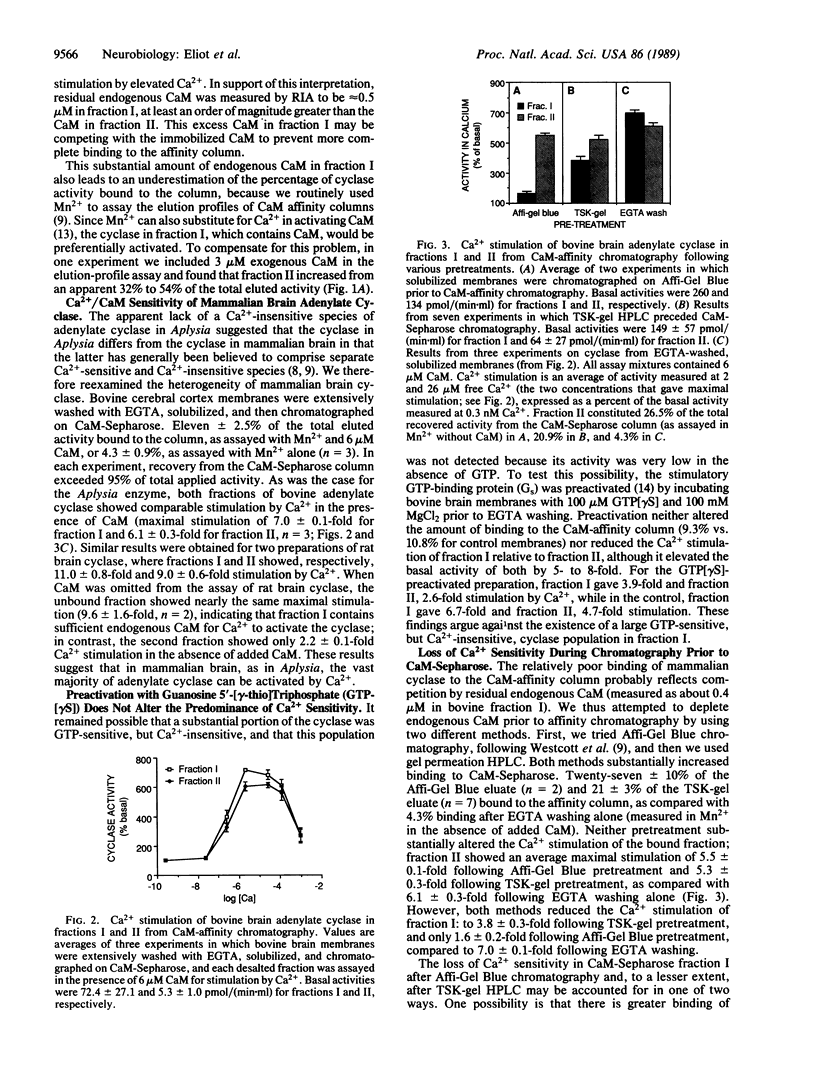
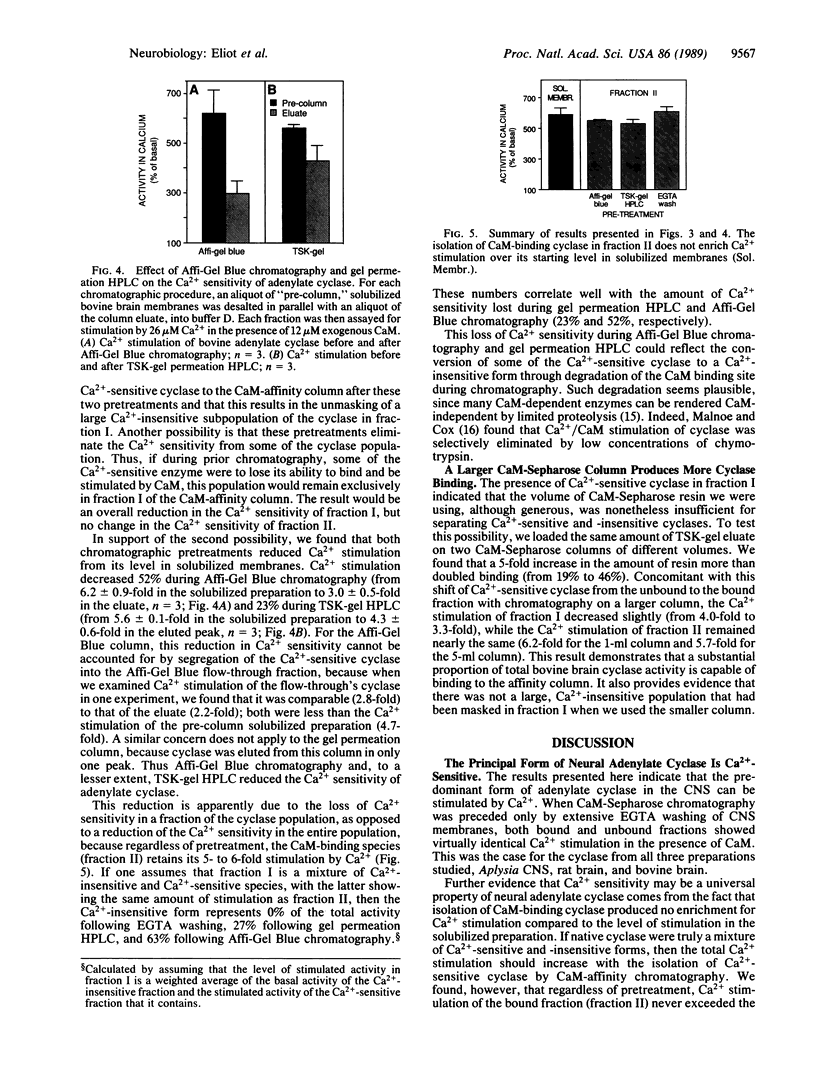
The Ca2+/calmodulin (CaM)-activated adenylate cyclase has been implicated as playing an important associative role in classical conditioning in both Aplysia and Drosophila. Studies of the cyclase in mammalian cerebral cortex have suggested that Ca2+/CaM sensitivity is confined to a subpopulation of total cyclase activity. We investigated the properties of cyclase from Aplysia, rat, and bovine central nervous system membranes by using CaM-Sepharose chromatography. Although only a minority of total cyclase activity bound to the CaM column, both bound and unbound fractions of cyclase from all three species showed comparable stimulation by Ca2+ in the presence of CaM. When solubilized bovine membranes were first depleted of most of their endogenous CaM by prior chromatography, binding to the CaM column was substantially increased and Ca2+ stimulation of the unbound fraction was somewhat reduced. However, this reduction in Ca2+ sensitivity resulted from the loss of Ca2+ sensitivity during prior chromatography, rather than from the more efficient separation of Ca2+-sensitive and -insensitive forms. This finding, together with the fact that we never observed any enrichment for Ca2+ sensitivity in the bound fraction over the level in the starting preparation, suggests that the vast majority of the cyclase present in solubilized central nervous system membranes is Ca2+/CaM-sensitive.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams T. W. Activity-dependent presynaptic facilitation: an associative mechanism in Aplysia. Cell Mol Neurobiol. 1985 Jun;5(1-2):123–145. doi: 10.1007/BF00711089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams T. W., Kandel E. R. Is contiguity detection in classical conditioning a system or a cellular property? Learning in Aplysia suggests a possible molecular site. Trends Neurosci. 1988 Apr;11(4):128–135. doi: 10.1016/0166-2236(88)90137-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Brostrom M. A., Wolff D. J. Calcium-dependent adenylate cyclase from rat cerebral cortex. Reversible activation by sodium fluoride. J Biol Chem. 1977 Aug 25;252(16):5677–5685. [PubMed] [Google Scholar]
- Dudai Y., Zvi S. Adenylate cyclase in the Drosophila memory mutant rutabaga displays an altered Ca2+ sensitivity. Neurosci Lett. 1984 Jun 15;47(2):119–124. doi: 10.1016/0304-3940(84)90416-6. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Abrams T., Bernier L., Carew T. J., Hawkins R. D., Schwartz J. H. Classical conditioning and sensitization share aspects of the same molecular cascade in Aplysia. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):821–830. doi: 10.1101/sqb.1983.048.01.085. [DOI] [PubMed] [Google Scholar]
- Krupinski J., Coussen F., Bakalyar H. A., Tang W. J., Feinstein P. G., Orth K., Slaughter C., Reed R. R., Gilman A. G. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science. 1989 Jun 30;244(4912):1558–1564. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Sziber P. P., Quinn W. G. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984 May;37(1):205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- MacNeil S., Lakey T., Tomlinson S. Calmodulin regulation of adenylate cyclase activity. Cell Calcium. 1985 Jun;6(3):213–216. doi: 10.1016/0143-4160(85)90007-7. [DOI] [PubMed] [Google Scholar]
- Malnoë A., Cox J. A. Relationship among calmodulin-, forskolin-, and guanine nucleotide-dependent adenylate cyclase activities in cerebellar membranes: studies by limited proteolysis. J Neurochem. 1985 Oct;45(4):1163–1171. doi: 10.1111/j.1471-4159.1985.tb05537.x. [DOI] [PubMed] [Google Scholar]
- Ocorr K. A., Walters E. T., Byrne J. H. Associative conditioning analog selectively increases cAMP levels of tail sensory neurons in Aplysia. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2548–2552. doi: 10.1073/pnas.82.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. B., Storm D. R. Immunological distinction between calmodulin-sensitive and calmodulin-insensitive adenylate cyclases. J Biol Chem. 1987 Jun 5;262(16):7623–7628. [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Toscano W. A., Jr, Westcott K. R., LaPorte D. C., Storm D. R. Evidence for a dissociable protein subunit required for calmodulin stimulation of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5582–5586. doi: 10.1073/pnas.76.11.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott K. R., La Porte D. C., Storm D. R. Resolution of adenylate cyclase sensitive and insensitive to Ca2+ and calcium-dependent regulatory protein (CDR) by CDR-sepharose affinity chromatography. Proc Natl Acad Sci U S A. 1979 Jan;76(1):204–208. doi: 10.1073/pnas.76.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff D. J., Poirier P. G., Brostrom C. O., Brostrom M. A. Divalent cation binding properties of bovine brain Ca2+-dependent regulator protein. J Biol Chem. 1977 Jun 25;252(12):4108–4117. [PubMed] [Google Scholar]



