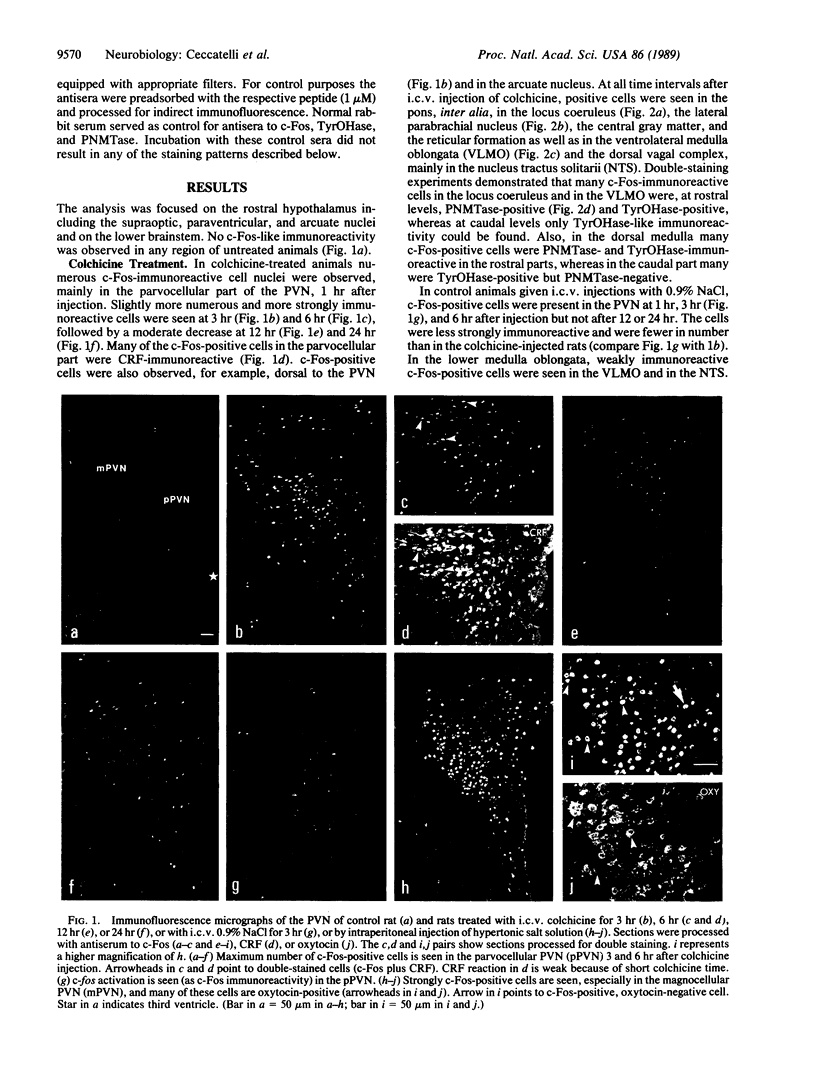
Abstract
The effect of intracerebroventricular injection of the mitosis inhibitor colchicine and of immobilization stress, subcutaneous injection of capsaicin, and intraperitoneal injection of hypertonic salt solution on expression of c-Fos-like immunoreactivity was studied in the rat brain with immunohistochemistry. All the procedures induced c-Fos immunoreactivity in parvocellular neurons of the paraventricular nucleus, and many of these neurons also contained corticotropin-releasing factor immunoreactivity. c-Fos immunoreactivity was also observed, for example, in subpopulations of neurons in the locus coeruleus, the ventrolateral medulla oblongata, and the nucleus tractus solitarii. Many of these cells also expressed catecholamine-synthesizing enzymes. The results suggest that intraventricular injection of colchicine is a stressful stimulus and support the view that several catecholamine cell groups in the lower brainstem are part of the brain circuitry mediating stress reactions, as are the hypothalamic neurons that contain corticotropin-releasing factor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnati L. F., Fuxe K., Yu Z. Y., Härfstrand A., Okret S., Wikström A. C., Goldstein M., Zoli M., Vale W., Gustafsson J. A. Morphometrical analysis of the distribution of corticotrophin releasing factor, glucocorticoid receptor and phenylethanolamine-N-methyltransferase immunoreactive structures in the paraventricular hypothalamic nucleus of the rat. Neurosci Lett. 1985 Mar 15;54(2-3):147–152. doi: 10.1016/s0304-3940(85)80070-7. [DOI] [PubMed] [Google Scholar]
- BARCHAS J. D., FREEDMAN D. X. BRAIN AMINES: RESPONSE TO PHYSIOLOGICAL STRESS. Biochem Pharmacol. 1963 Oct;12:1232–1235. doi: 10.1016/0006-2952(63)90101-1. [DOI] [PubMed] [Google Scholar]
- Barry J., Dubois M. P., Poulain P. LRF producing cells of the mammalian hypothalamus. A fluorescent antibody study. Z Zellforsch Mikrosk Anat. 1973 Dec 31;146(3):351–366. doi: 10.1007/BF02346227. [DOI] [PubMed] [Google Scholar]
- Ben-Barak Y., Russell J. T., Whitnall M. H., Ozato K., Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. I. Production and characterization of monoclonal antibodies. J Neurosci. 1985 Jan;5(1):81–97. doi: 10.1523/JNEUROSCI.05-01-00081.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss E. L., Ailion J., Zwanziger J. Metabolism of norepinephrine, serotonin and dopamine in rat brain with stress. J Pharmacol Exp Ther. 1968 Nov;164(1):122–134. [PubMed] [Google Scholar]
- Ceccatelli S., Eriksson M., Hökfelt T. Distribution and coexistence of corticotropin-releasing factor-, neurotensin-, enkephalin-, cholecystokinin-, galanin- and vasoactive intestinal polypeptide/peptide histidine isoleucine-like peptides in the parvocellular part of the paraventricular nucleus. Neuroendocrinology. 1989 Mar;49(3):309–323. doi: 10.1159/000125133. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Corrodi H., Fuxe K., Hökfelt T. The effect of immobilization stress on the activity of central monoamine neurons. Life Sci. 1968 Jan 1;7(1):107–112. doi: 10.1016/0024-3205(68)90368-8. [DOI] [PubMed] [Google Scholar]
- Curran T., MacConnell W. P., van Straaten F., Verma I. M. Structure of the FBJ murine osteosarcoma virus genome: molecular cloning of its associated helper virus and the cellular homolog of the v-fos gene from mouse and human cells. Mol Cell Biol. 1983 May;3(5):914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science. 1985 Sep 20;229(4719):1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- Dahlström A. Effect of colchicine on transport of amine storage granules in sympathetic nerves of rat. Eur J Pharmacol. 1968 Dec;5(1):111–113. doi: 10.1016/0014-2999(68)90165-9. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Robertson H. A. Kindling stimulation induces c-fos protein(s) in granule cells of the rat dentate gyrus. Nature. 1987 Oct 1;329(6138):441–442. doi: 10.1038/329441a0. [DOI] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Goldstein M., Fuxe K., Hökfelt T. Characterization and tissue localization of catecholamine synthesizing enzymes. Pharmacol Rev. 1972 Jun;24(2):293–309. [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hamamura M., Shibuki K., Yagi K. Noxious inputs to supraoptic neurosecretory cells in the rat. Neurosci Res. 1984 Dec;2(1-2):49–61. doi: 10.1016/0168-0102(84)90004-x. [DOI] [PubMed] [Google Scholar]
- Hunt S. P., Pini A., Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987 Aug 13;328(6131):632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Härfstrand A., Fuxe K., Cintra A., Agnati L. F., Zini I., Wikström A. C., Okret S., Yu Z. Y., Goldstein M., Steinbusch H. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Dahlström A. Effects of two mitosis inhibitors (colchicine and vinblastine) on the distribution and axonal transport of noradrenaline storage particles, studied by fluorescence and electron microscopy. Z Zellforsch Mikrosk Anat. 1971;119(4):460–482. doi: 10.1007/BF00455243. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res. 1975 Dec 19;100(2):235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- Korf J., Aghajanian G. K., Roth R. H. Increased turnover of norepinephrine in the rat cerebral cortex during stress: role of the locus coeruleus. Neuropharmacology. 1973 Oct;12(10):933–938. doi: 10.1016/0028-3908(73)90024-5. [DOI] [PubMed] [Google Scholar]
- Kreutzberg G. W. Neuronal dynamics and axonal flow. IV. Blockage of intra-axonal enzyme transport by colchicine. Proc Natl Acad Sci U S A. 1969 Mar;62(3):722–728. doi: 10.1073/pnas.62.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvetnanský R., Palkovits M., Mitro A., Torda T., Mikulaj L. Catecholamines in individual hypothalamic nuclei of acutely and repeatedly stressed rats. Neuroendocrinology. 1977;23(5):257–267. doi: 10.1159/000122673. [DOI] [PubMed] [Google Scholar]
- Kvetñanský R., Kopin I. J., Saavedra J. M. Changes in epinephrine in individual hypothalamic nuclei after immobilization stress. Brain Res. 1978 Oct 27;155(2):387–390. doi: 10.1016/0006-8993(78)91035-1. [DOI] [PubMed] [Google Scholar]
- MAYNERT E. W., LEVI R. STRESS-INDUCED RELEASE OF BRAIN NOREPINEPHRINE AND ITS INHIBITION BY DRUGS. J Pharmacol Exp Ther. 1964 Jan;143:90–95. [PubMed] [Google Scholar]
- MOORE K. E., LARIVIERE E. W. EFFECTS OF STRESS AND D-AMPHETAMINE ON RAT BRAIN CATECHOLAMINES. Biochem Pharmacol. 1964 Jul;13:1098–1100. doi: 10.1016/0006-2952(64)90107-8. [DOI] [PubMed] [Google Scholar]
- Markey K. A., Kondo H., Shenkman L., Goldstein M. Purification and characterization of tyrosine hydroxylase from a clonal pheochromocytoma cell line. Mol Pharmacol. 1980 Jan;17(1):79–85. [PubMed] [Google Scholar]
- Menétrey D., Gannon A., Levine J. D., Basbaum A. I. Expression of c-fos protein in interneurons and projection neurons of the rat spinal cord in response to noxious somatic, articular, and visceral stimulation. J Comp Neurol. 1989 Jul 8;285(2):177–195. doi: 10.1002/cne.902850203. [DOI] [PubMed] [Google Scholar]
- Mezey E., Kiss J. Z., Skirboll L. R., Goldstein M., Axelrod J. Increase of corticotropin-releasing factor staining in rat paraventricular nucleus neurones by depletion of hypothalamic adrenaline. Nature. 1984 Jul 12;310(5973):140–141. doi: 10.1038/310140a0. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987 Jul 10;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Musacchio J. M., Julou L., Kety S. S., Glowinski J. Increase in rat brain tyrosine hydroxylase activity produced by electroconvulsive shock. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1117–1119. doi: 10.1073/pnas.63.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Onaka T., Hamamura M., Yagi K. Potentiation of vasopressin secretion by footshocks in rats. Jpn J Physiol. 1986;36(6):1253–1260. doi: 10.2170/jjphysiol.36.1253. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Kobayashi R. M., Kizer J. S., Jacobowitz D. M., Kopin I. J. Effects of stress on catecholamines and tyrosine hydroxylase activity of individual hypothalamic nuclei. Neuroendocrinology. 1975;18(2):144–153. doi: 10.1159/000122394. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M., Kvetnansky R., Kopin I. J. Adrenaline, noradrenaline and dopamine levels in specific brain stem areas of acutely immobilized rats. Brain Res. 1979 Jan 12;160(2):271–280. doi: 10.1016/0006-8993(79)90424-4. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M., Torda T. Increased brain stem and decreased hypothalamic adrenaline-forming enzyme after acute and repeated immobilization stress in the rat. Neuroendocrinology. 1980 Aug;31(2):142–146. doi: 10.1159/000123065. [DOI] [PubMed] [Google Scholar]
- Sagar S. M., Sharp F. R., Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988 Jun 3;240(4857):1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Vale W. W. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1883–1887. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K., Onaka T., Hamamura M., Yagi K., Ishikawa S., Saito T., Yoshida S. Synergistic interactions between footshocks and non-osmotic hypovolemia on vasopressin secretion in rats. Brain Res. 1987 Apr 28;410(1):140–142. doi: 10.1016/s0006-8993(87)80035-5. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36(3):165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Thierry A. M., Javoy F., Glowinski J., Kety S. S. Effects of stress on the metabolism of norepinephrine, dopamine and serotonin in the central nervous system of the rat. I. Modifications of norepinephrine turnover. J Pharmacol Exp Ther. 1968 Sep;163(1):163–171. [PubMed] [Google Scholar]
- Turner B. B., Katz R. J., Roth K. A., Carroll B. J. Central elevation of phenylethanolamine N-methyltransferase activity following stress. Brain Res. 1978 Sep 22;153(2):419–422. doi: 10.1016/0006-8993(78)90426-2. [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Zigmond R. E., Schon F., Iversen L. L. Increased tyrosine hydroxylase activity in the locus coeruleus of rat brain stem after reserpine treatment and cold stress. Brain Res. 1974 Apr 26;70(3):547–552. doi: 10.1016/0006-8993(74)90267-4. [DOI] [PubMed] [Google Scholar]