Abstract
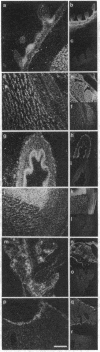
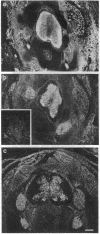
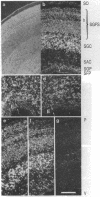
The differential expression of the mRNA for the neural cell adhesion molecule (N-CAM) has been studied by in situ hybridization and compared with protein localization analyzed by immunohistochemical staining. The distribution of mRNA during chicken embryonic development was analyzed in neural and nonneural tissues by using an RNA probe that detects all N-CAM mRNAs and a probe specific for the mRNA of the large cytoplasmic domain (ld) of N-CAM. The results provide a detailed description of the mRNA distribution for N-CAM. The distribution of mRNA for total N-CAM generally corresponded to that of protein but differed at a more detailed level of analysis. For example, the mRNA was localized only within the cell bodies of neurons, whereas the protein was also in neuronal processes; this differential localization was most clearly seen in the alternating layers of cell bodies and fibers in the optic tectum and cerebellum. N-CAM ld mRNA, which arises from alternative RNA splicing, was expressed only in neural tissues, confirming previous biochemical and histological studies. Differential expression of the ld mRNA was detected in specific neural cell types: N-CAM mRNA was present in the ependymal cells of the spinal cord and optic tectum, but mRNA for the ld form was absent. In contrast, the ld mRNA was among the N-CAM mRNAs found in the Purkinje cells and internal granule cells in the cerebellum. The differential expression of mRNAs for the N-CAM forms emphasizes the potential importance of alternative mRNA splicing in modulating adhesive events during embryonic development, particularly in the nervous system.
Full text
PDF


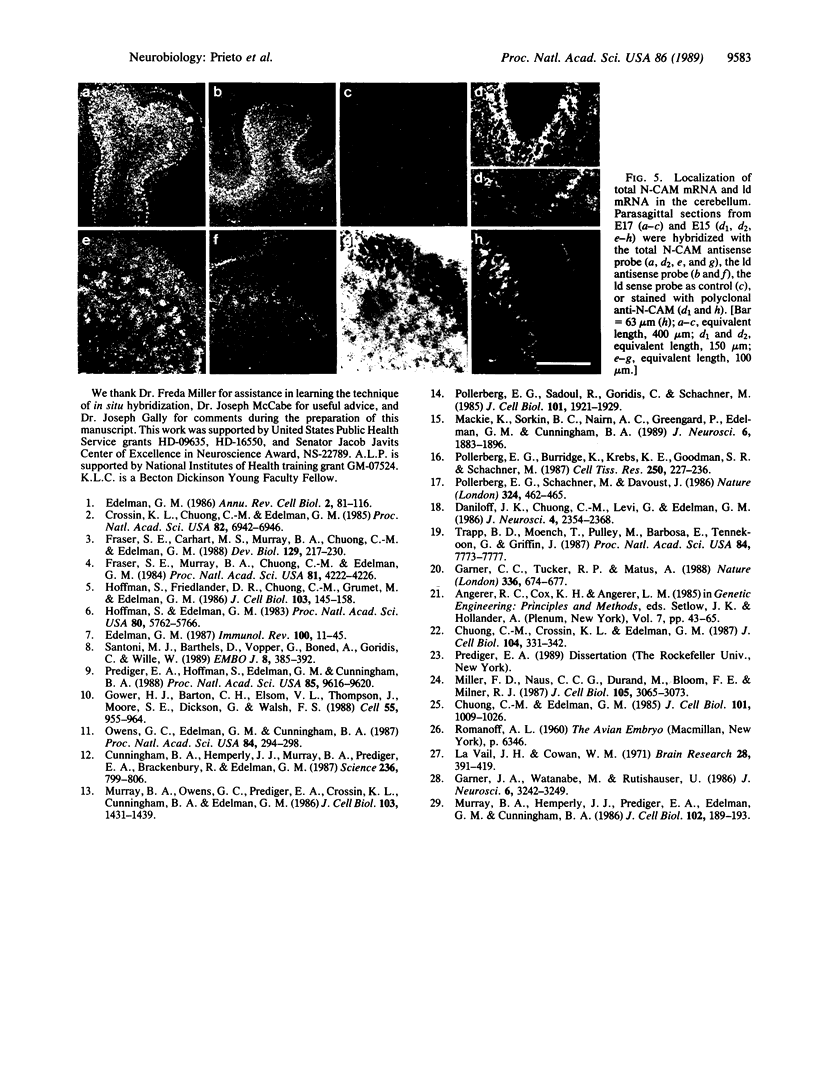
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chuong C. M., Crossin K. L., Edelman G. M. Sequential expression and differential function of multiple adhesion molecules during the formation of cerebellar cortical layers. J Cell Biol. 1987 Feb;104(2):331–342. doi: 10.1083/jcb.104.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C. M., Edelman G. M. Expression of cell-adhesion molecules in embryonic induction. I. Morphogenesis of nestling feathers. J Cell Biol. 1985 Sep;101(3):1009–1026. doi: 10.1083/jcb.101.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L., Chuong C. M., Edelman G. M. Expression sequences of cell adhesion molecules. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6942–6946. doi: 10.1073/pnas.82.20.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. CAMs and Igs: cell adhesion and the evolutionary origins of immunity. Immunol Rev. 1987 Dec;100:11–45. doi: 10.1111/j.1600-065x.1987.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Fraser S. E., Carhart M. S., Murray B. A., Chuong C. M., Edelman G. M. Alterations in the Xenopus retinotectal projection by antibodies to Xenopus N-CAM. Dev Biol. 1988 Sep;129(1):217–230. doi: 10.1016/0012-1606(88)90176-5. [DOI] [PubMed] [Google Scholar]
- Fraser S. E., Murray B. A., Chuong C. M., Edelman G. M. Alteration of the retinotectal map in Xenopus by antibodies to neural cell adhesion molecules. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4222–4226. doi: 10.1073/pnas.81.13.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C. C., Tucker R. P., Matus A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature. 1988 Dec 15;336(6200):674–677. doi: 10.1038/336674a0. [DOI] [PubMed] [Google Scholar]
- Garner J. A., Watanabe M., Rutishauser U. Rapid axonal transport of the neural cell adhesion molecule. J Neurosci. 1986 Nov;6(11):3242–3249. doi: 10.1523/JNEUROSCI.06-11-03242.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower H. J., Barton C. H., Elsom V. L., Thompson J., Moore S. E., Dickson G., Walsh F. S. Alternative splicing generates a secreted form of N-CAM in muscle and brain. Cell. 1988 Dec 23;55(6):955–964. doi: 10.1016/0092-8674(88)90241-3. [DOI] [PubMed] [Google Scholar]
- Hoffman S., Edelman G. M. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5762–5766. doi: 10.1073/pnas.80.18.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Friedlander D. R., Chuong C. M., Grumet M., Edelman G. M. Differential contributions of Ng-CAM and N-CAM to cell adhesion in different neural regions. J Cell Biol. 1986 Jul;103(1):145–158. doi: 10.1083/jcb.103.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail J. H., Cowan W. M. The development of the chick optic tectum. I. Normal morphology and cytoarchitectonic development. Brain Res. 1971 May 21;28(3):391–419. doi: 10.1016/0006-8993(71)90053-9. [DOI] [PubMed] [Google Scholar]
- Mackie K., Sorkin B. C., Nairn A. C., Greengard P., Edelman G. M., Cunningham B. A. Identification of two protein kinases that phosphorylate the neural cell-adhesion molecule, N-CAM. J Neurosci. 1989 Jun;9(6):1883–1896. doi: 10.1523/JNEUROSCI.09-06-01883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. D., Naus C. C., Durand M., Bloom F. E., Milner R. J. Isotypes of alpha-tubulin are differentially regulated during neuronal maturation. J Cell Biol. 1987 Dec;105(6 Pt 2):3065–3073. doi: 10.1083/jcb.105.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. A., Hemperly J. J., Prediger E. A., Edelman G. M., Cunningham B. A. Alternatively spliced mRNAs code for different polypeptide chains of the chicken neural cell adhesion molecule (N-CAM). J Cell Biol. 1986 Jan;102(1):189–193. doi: 10.1083/jcb.102.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. A., Owens G. C., Prediger E. A., Crossin K. L., Cunningham B. A., Edelman G. M. Cell surface modulation of the neural cell adhesion molecule resulting from alternative mRNA splicing in a tissue-specific developmental sequence. J Cell Biol. 1986 Oct;103(4):1431–1439. doi: 10.1083/jcb.103.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. C., Edelman G. M., Cunningham B. A. Organization of the neural cell adhesion molecule (N-CAM) gene: alternative exon usage as the basis for different membrane-associated domains. Proc Natl Acad Sci U S A. 1987 Jan;84(1):294–298. doi: 10.1073/pnas.84.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollerberg E. G., Sadoul R., Goridis C., Schachner M. Selective expression of the 180-kD component of the neural cell adhesion molecule N-CAM during development. J Cell Biol. 1985 Nov;101(5 Pt 1):1921–1929. doi: 10.1083/jcb.101.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollerberg G. E., Burridge K., Krebs K. E., Goodman S. R., Schachner M. The 180-kD component of the neural cell adhesion molecule N-CAM is involved in cell-cell contacts and cytoskeleton-membrane interactions. Cell Tissue Res. 1987 Oct;250(1):227–236. doi: 10.1007/BF00214676. [DOI] [PubMed] [Google Scholar]
- Pollerberg G. E., Schachner M., Davoust J. Differentiation state-dependent surface mobilities of two forms of the neural cell adhesion molecule. Nature. 1986 Dec 4;324(6096):462–465. doi: 10.1038/324462a0. [DOI] [PubMed] [Google Scholar]
- Prediger E. A., Hoffman S., Edelman G. M., Cunningham B. A. Four exons encode a 93-base-pair insert in three neural cell adhesion molecule mRNAs specific for chicken heart and skeletal muscle. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9616–9620. doi: 10.1073/pnas.85.24.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni M. J., Barthels D., Vopper G., Boned A., Goridis C., Wille W. Differential exon usage involving an unusual splicing mechanism generates at least eight types of NCAM cDNA in mouse brain. EMBO J. 1989 Feb;8(2):385–392. doi: 10.1002/j.1460-2075.1989.tb03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B. D., Moench T., Pulley M., Barbosa E., Tennekoon G., Griffin J. Spatial segregation of mRNA encoding myelin-specific proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7773–7777. doi: 10.1073/pnas.84.21.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]