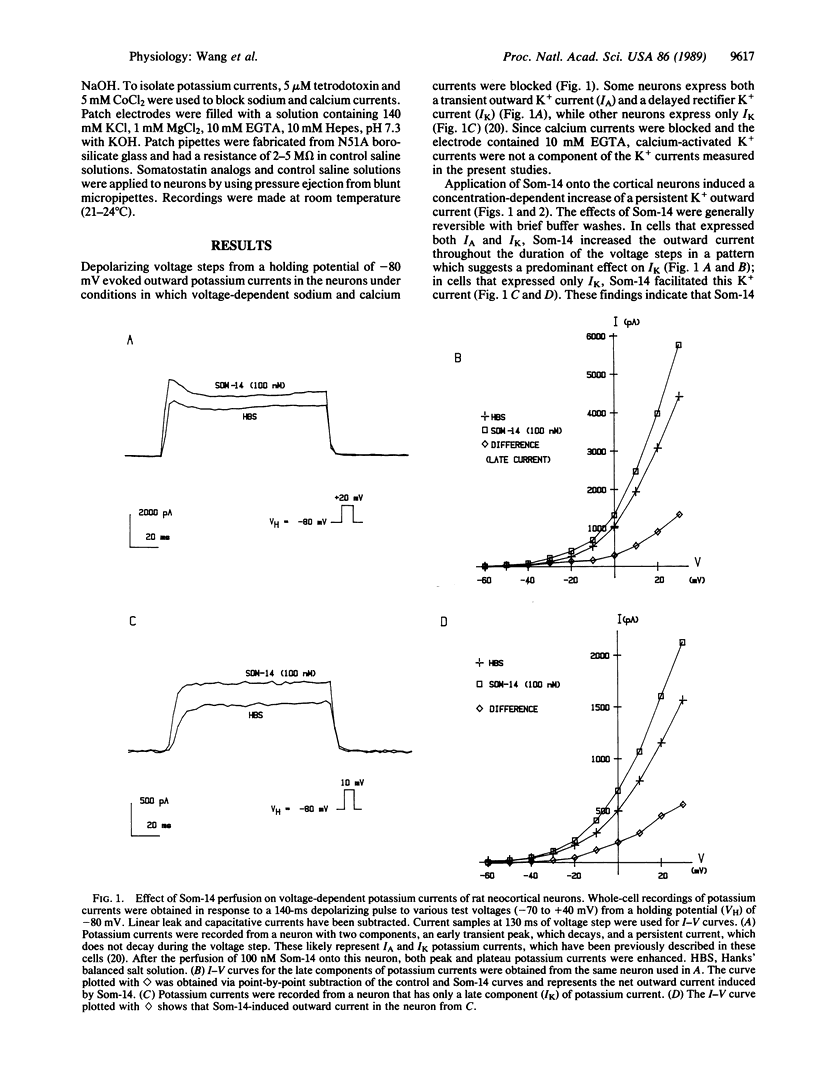
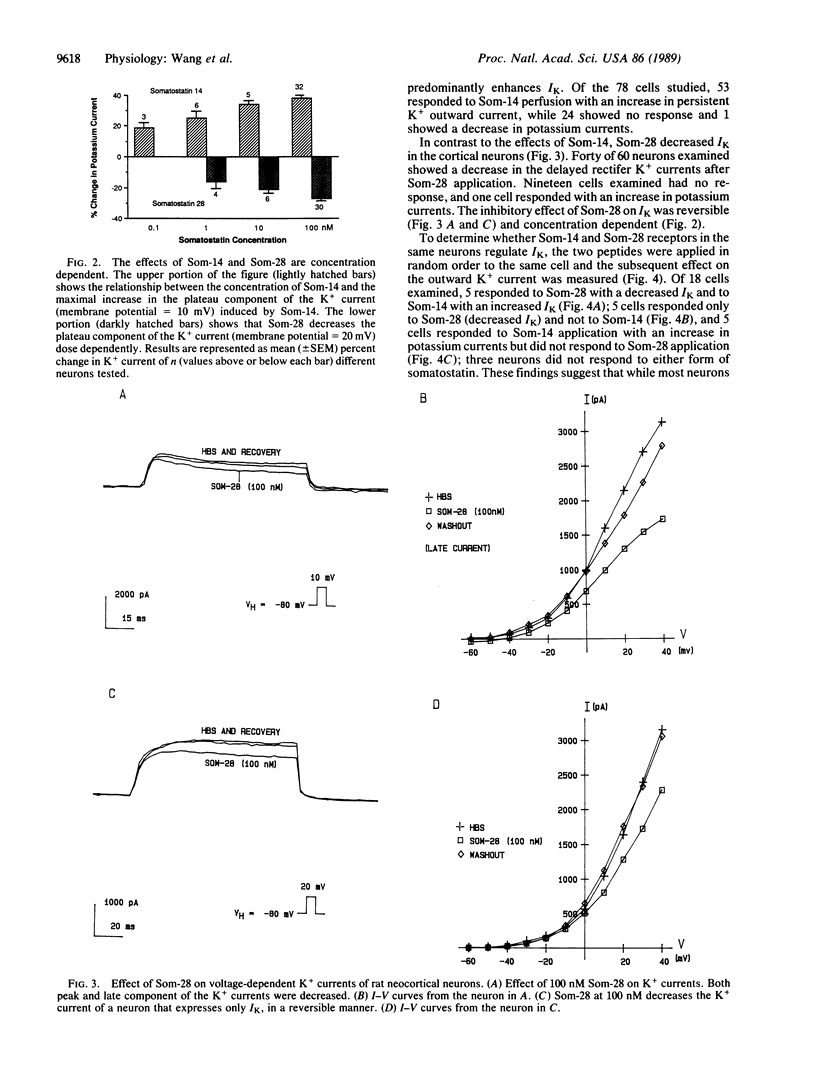
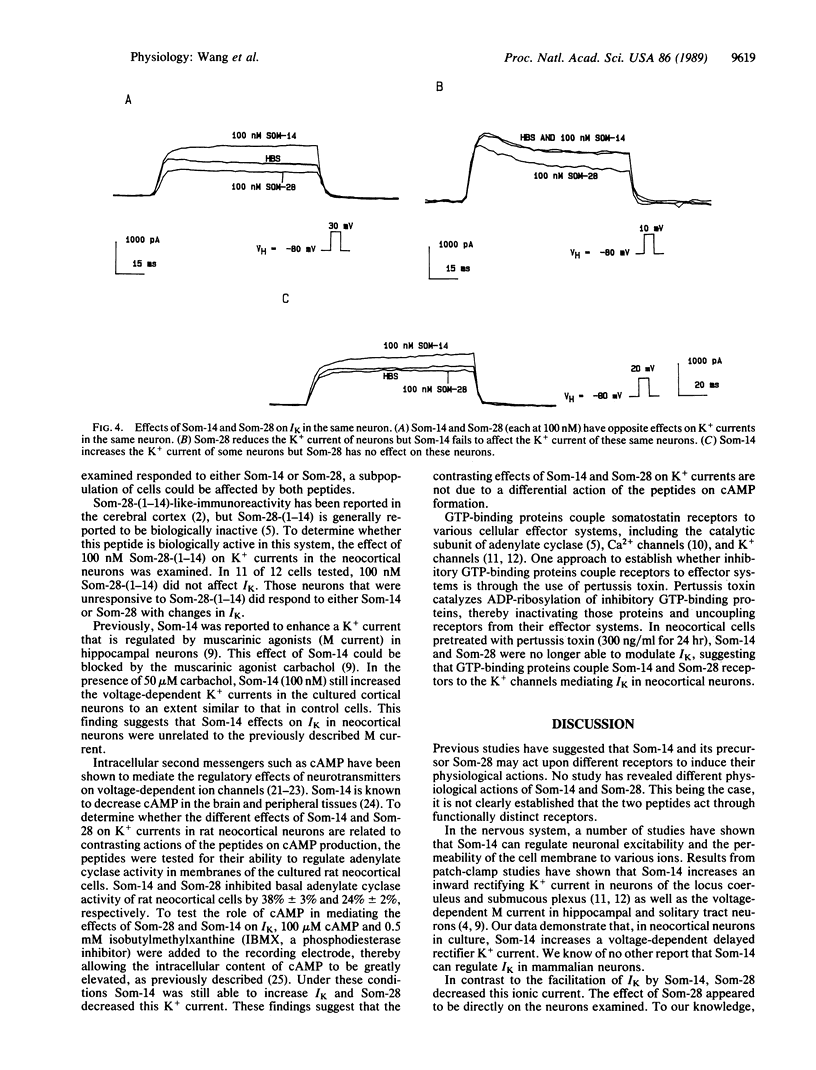
Abstract
The prosomatostatin-derived peptides somatostatin-14 (Som-14) and somatostatin-28 (Som-28) are believed to act as neurotransmitters in the central nervous system. To examine possible mechanisms by which these peptides induce their physiological actions in brain, the effects of Som-14 and Som-28 on voltage-dependent K+ currents in rat cerebral cortical neurons in culture were examined by using whole-cell patch-clamp techniques. Som-14 increased a delayed rectifier K+ current (IK) in the cortical neurons, while Som-28 reduced IK in the neurons, both in a concentration-dependent manner. Som-14 and Som-28 could induce opposite changes in IK in the same neurons. Elevating intracellular cAMP in the cortical neurons did not modify the effects of Som-14 or Som-28 on IK, indicating that the peptides can regulate this ionic current through cAMP-independent mechanisms. Pretreatment of the neocortical cells with pertussis toxin, which inactivates inhibitory GTP-binding proteins, abolished both Som-14 and Som-28 modulation of IK, indicating that Som-14 and Som-28 receptors are coupled to IK via GTP-binding proteins. These studies show that Som-14 and Som-28 can induce opposite biological effects, suggesting that Som-14 and Som-28, acting through distinct receptors, may function as different neurotransmitters or neuromodulators.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M., Rivier J., Vale W. Somatostatin-28: selective action on the pancreatic beta-cell and brain. Endocrinology. 1981 Jun;108(6):2391–2396. doi: 10.1210/endo-108-6-2391. [DOI] [PubMed] [Google Scholar]
- Chesselet M. F., Reisine T. D. Somatostatin regulates dopamine release in rat striatal slices and cat caudate nuclei. J Neurosci. 1983 Jan;3(1):232–236. doi: 10.1523/JNEUROSCI.03-01-00232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chneiweiss H., Glowinski J., Prémont J. Modulation by monoamines of somatostatin-sensitive adenylate cyclase on neuronal and glial cells from the mouse brain in primary cultures. J Neurochem. 1985 Jun;44(6):1825–1831. doi: 10.1111/j.1471-4159.1985.tb07175.x. [DOI] [PubMed] [Google Scholar]
- Delfs J., Robbins R., Connolly J. L., Dichter M., Reichlin S. Somatostatin production by rat cerebral neurones in dissociated cell culture. Nature. 1980 Feb 14;283(5748):676–677. doi: 10.1038/283676a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Muscarinic control of the hyperpolarization-activated current (if) in rabbit sino-atrial node myocytes. J Physiol. 1988 Nov;405:493–510. doi: 10.1113/jphysiol.1988.sp017344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M. A. Rat cortical neurons in cell culture: culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 1978 Jun 30;149(2):279–293. doi: 10.1016/0006-8993(78)90476-6. [DOI] [PubMed] [Google Scholar]
- Epelbaum J. Somatostatin in the central nervous system: physiology and pathological modifications. Prog Neurobiol. 1986;27(1):63–100. doi: 10.1016/0301-0082(86)90012-2. [DOI] [PubMed] [Google Scholar]
- Gluschankof P., Morel A., Gomez S., Nicolas P., Fahy C., Cohen P. Enzymes processing somatostatin precursors: an Arg-Lys esteropeptidase from the rat brain cortex converting somatostatin-28 into somatostatin-14. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6662–6666. doi: 10.1073/pnas.81.21.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R., Johnston D. Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987 Jun 18;327(6123):620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G. Somatostatin blocks a calcium current in rat sympathetic ganglion neurones. J Physiol. 1989 Feb;409:221–240. doi: 10.1113/jphysiol.1989.sp017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Nakajima S., Nakajima Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J Physiol. 1988 Dec;407:177–198. doi: 10.1113/jphysiol.1988.sp017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin T., Champagnat J., Madamba S., Denavit-Saubié M., Siggins G. R. Somatostatin depresses excitability in neurons of the solitary tract complex through hyperpolarization and augmentation of IM, a non-inactivating voltage-dependent outward current blocked by muscarinic agonists. Proc Natl Acad Sci U S A. 1988 Feb;85(3):948–952. doi: 10.1073/pnas.85.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson O., Hökfelt T., Elde R. P. Immunohistochemical distribution of somatostatin-like immunoreactivity in the central nervous system of the adult rat. Neuroscience. 1984 Oct;13(2):265–339. doi: 10.1016/0306-4522(84)90233-1. [DOI] [PubMed] [Google Scholar]
- Lechleiter J. D., Dartt D. A., Brehm P. Vasoactive intestinal peptide activates Ca2(+)-dependent K+ channels through a cAMP pathway in mouse lacrimal cells. Neuron. 1988 May;1(3):227–235. doi: 10.1016/0896-6273(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Iversen L. L. Release of somatostatin from extra-hypothalamic rat brain slices: inhibition by dopamine and morphine. Brain Res. 1981 Aug 31;219(2):355–361. doi: 10.1016/0006-8993(81)90298-5. [DOI] [PubMed] [Google Scholar]
- Leroux P., Quirion R., Pelletier G. Localization and characterization of brain somatostatin receptors as studied with somatostatin-14 and somatostatin-28 receptor radioautography. Brain Res. 1985 Nov 11;347(1):74–84. doi: 10.1016/0006-8993(85)90890-x. [DOI] [PubMed] [Google Scholar]
- Mandarino L., Stenner D., Blanchard W., Nissen S., Gerich J., Ling N., Brazeau P., Bohlen P., Esch F., Guillemin R. Selective effects of somatostatin-14, -25 and -28 on in vitro insulin and glucagon secretion. Nature. 1981 May 7;291(5810):76–77. doi: 10.1038/291076a0. [DOI] [PubMed] [Google Scholar]
- Mihara S., North R. A., Surprenant A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurones. J Physiol. 1987 Sep;390:335–355. doi: 10.1113/jphysiol.1987.sp016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. D., Madamba S. G., Joëls M., Siggins G. R. Somatostatin augments the M-current in hippocampal neurons. Science. 1988 Jan 15;239(4837):278–280. doi: 10.1126/science.2892268. [DOI] [PubMed] [Google Scholar]
- Morrison J. H., Benoit R., Magistretti P. J., Bloom F. E. Immunohistochemical distribution of pro-somatostatin-related peptides in cerebral cortex. Brain Res. 1983 Mar 7;262(2):344–351. doi: 10.1016/0006-8993(83)91031-4. [DOI] [PubMed] [Google Scholar]
- Srikant C. B., Patel Y. C. Somatostatin receptors: identification and characterization in rat brain membranes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3930–3934. doi: 10.1073/pnas.78.6.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermos K., He H. T., Wang H. L., Margolis N., Reisine T. Biochemical properties of brain somatostatin receptors. Neuroscience. 1989;31(1):131–141. doi: 10.1016/0306-4522(89)90035-3. [DOI] [PubMed] [Google Scholar]
- Thermos K., Reisine T. Somatostatin receptor subtypes in the clonal anterior pituitary cell lines AtT-20 and GH3. Mol Pharmacol. 1988 Apr;33(4):370–377. [PubMed] [Google Scholar]
- Zona C., Pirrone G., Avoli M., Dichter M. Delayed and fast transient potassium currents in rat neocortical neurons in cell culture. Neurosci Lett. 1988 Dec 5;94(3):285–290. doi: 10.1016/0304-3940(88)90032-8. [DOI] [PubMed] [Google Scholar]



