Abstract
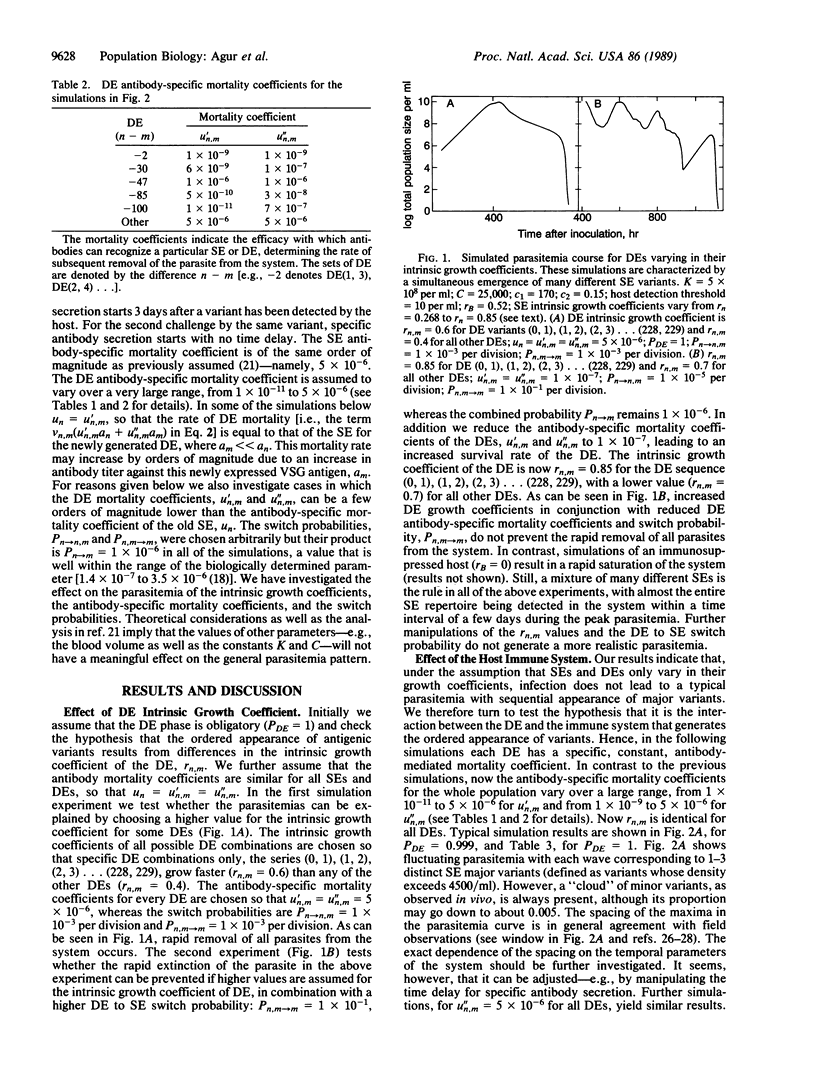
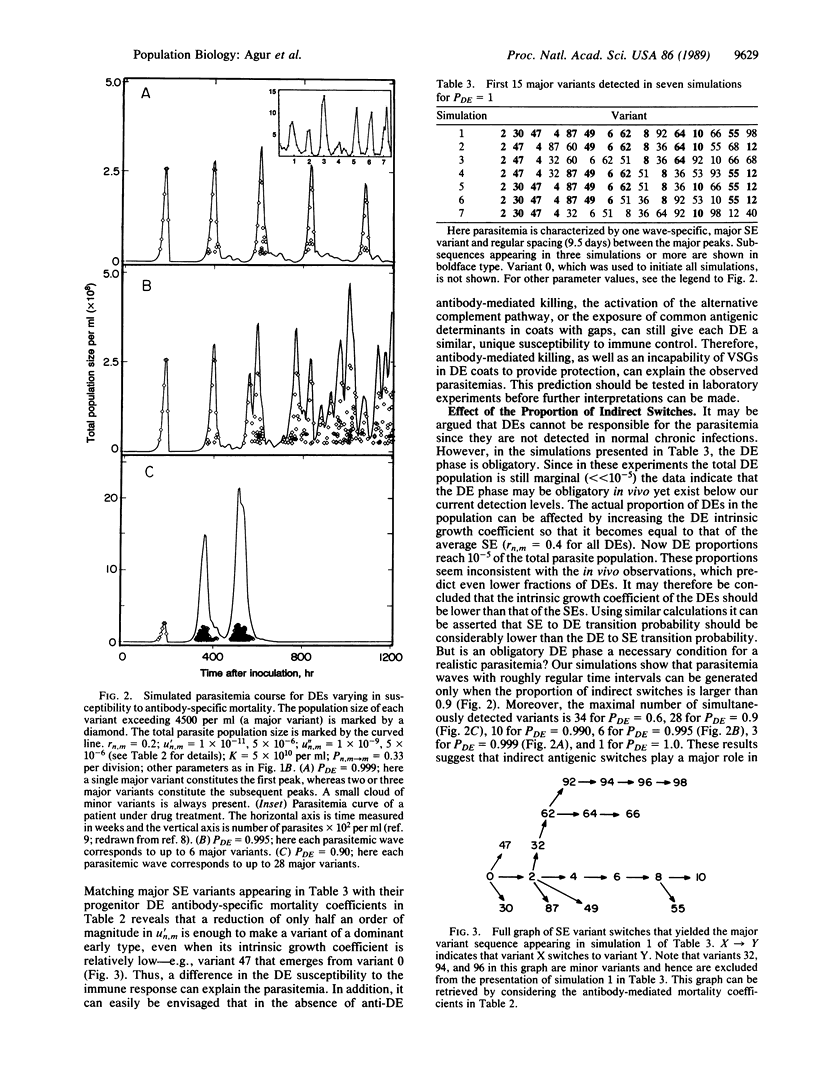
Antigenic variation of African trypanosomes results from the periodic activation of a single new variant cell surface glycoprotein (VSG) gene out of a repertoire of about a 1000 VSG genes. In spite of the apparently random genetic basis of the process of antigenic variation, the relapsing parasitemias are characterized by an as yet unexplained order of appearance of major VSG variants. Here we mathematically test hypotheses concerning the blood-based parasitemia. In our model the antigenic switches occur at random at the DNA level. A variable proportion of the switches has a short intermediate phase in which two different VSGs simultaneously occur on the cell surface. We show that, in a theoretical population of 230 single expressor variants in an immunocompetent or in an immunodeficient host, it is not possible to explain the ordered appearance of variants by affecting the growth coefficients of single expressors or double expressors or by affecting the antigen switch probabilities. Rather, a realistic parasitemia can be obtained if the majority of switches has a double expressor switch-intermediate phase and if the double expressors have a differential susceptibility to the immune control. This study is significant in providing a theoretical basis for the ordered appearance of variants and in explaining previously unresolved discrepancies between the rate of appearance of new variants in culture and in vivo. In addition, testable predictions as to the development of the infections, switch rate of variants, fraction of double expressors, and parasite mortality coefficients are generated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aline R., Jr, MacDonald G., Brown E., Allison J., Myler P., Rothwell V., Stuart K. (TAA)n within sequences flanking several intrachromosomal variant surface glycoprotein genes in Trypanosoma brucei. Nucleic Acids Res. 1985 May 10;13(9):3161–3177. doi: 10.1093/nar/13.9.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz T., Giroud C., Baltz D., Roth C., Raibaud A., Eisen H. Stable expression of two variable surface glycoproteins by cloned Trypanosoma equiperdum. Nature. 1986 Feb 13;319(6054):602–604. doi: 10.1038/319602a0. [DOI] [PubMed] [Google Scholar]
- Bernards A. Antigenic variation of trypanosomes. Biochim Biophys Acta. 1985 Jan 29;824(1):1–15. doi: 10.1016/0167-4781(85)90023-5. [DOI] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Capbern A., Giroud C., Baltz T., Mattern P. Trypanosoma equiperdum: etude des variations antigéniques au cours de la trypanosomose experimentale du lapin. Exp Parasitol. 1977 Jun;42(1):6–13. doi: 10.1016/0014-4894(77)90055-8. [DOI] [PubMed] [Google Scholar]
- Cornelissen A. W., Johnson P. J., Kooter J. M., Van der Ploeg L. H., Borst P. Two simultaneously active VSG gene transcription units in a single Trypanosoma brucei variant. Cell. 1985 Jul;41(3):825–832. doi: 10.1016/s0092-8674(85)80063-5. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- Doyle J. J., Hirumi H., Hirumi K., Lupton E. N., Cross G. A. Antigenic variation in clones of animal-infective Trypanosoma brucei derived and maintained in vitro. Parasitology. 1980 Apr;80(2):359–369. doi: 10.1017/s0031182000000810. [DOI] [PubMed] [Google Scholar]
- Esser K. M., Schoenbechler M. J. Expression of two variant surface glycoproteins on individual African trypanosomes during antigen switching. Science. 1985 Jul 12;229(4709):190–193. doi: 10.1126/science.3892689. [DOI] [PubMed] [Google Scholar]
- Herbert W. J. Trypanosoma brucei: the mechanism of remission in murine infections. A calculator simulation. Parasite Immunol. 1982 May;4(3):209–217. doi: 10.1111/j.1365-3024.1982.tb00432.x. [DOI] [PubMed] [Google Scholar]
- Hirumi H., Hirumi K., Doyle J. J., Cross G. A. In vitro cloning of animal-infective bloodstream forms of Trypanosoma brucei. Parasitology. 1980 Apr;80(2):371–382. doi: 10.1017/s0031182000000822. [DOI] [PubMed] [Google Scholar]
- Kosinski R. J. Antigenic variation in trypanosomes: a computer analysis of variant order. Parasitology. 1980 Apr;80(2):343–357. doi: 10.1017/s0031182000000809. [DOI] [PubMed] [Google Scholar]
- Lamont G. S., Tucker R. S., Cross G. A. Analysis of antigen switching rates in Trypanosoma brucei. Parasitology. 1986 Apr;92(Pt 2):355–367. doi: 10.1017/s003118200006412x. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Van der Ploeg L. H. Frequent independent duplicative transpositions activate a single VSG gene. Mol Cell Biol. 1987 Jan;7(1):357–364. doi: 10.1128/mcb.7.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. N., Turner M. J. Analysis of antigenic types appearing in first relapse populations of clones of Trypanosoma brucei. Parasitology. 1981 Feb;82(1):63–80. doi: 10.1017/s0031182000041871. [DOI] [PubMed] [Google Scholar]
- Myler P., Nelson R. G., Agabian N., Stuart K. Two mechanisms of expression of a predominant variant antigen gene of Trypanosoma brucei. Nature. 1984 May 17;309(5965):282–284. doi: 10.1038/309282a0. [DOI] [PubMed] [Google Scholar]
- Seed J. R. Competition among serologically different clones of Trypanosoma brucei gambiense in vivo. J Protozool. 1978 Nov;25(4):526–529. doi: 10.1111/j.1550-7408.1978.tb04179.x. [DOI] [PubMed] [Google Scholar]
- Seed J. R., Edwards R., Sechelski J. The ecology of antigenic variation. J Protozool. 1984 Feb;31(1):48–53. doi: 10.1111/j.1550-7408.1984.tb04288.x. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H. Control of variant surface antigen switching in trypanosomes. Cell. 1987 Oct 23;51(2):159–161. doi: 10.1016/0092-8674(87)90140-1. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Valerio D., De Lange T., Bernards A., Borst P., Grosveld F. G. An analysis of cosmid clones of nuclear DNA from Trypanosoma brucei shows that the genes for variant surface glycoproteins are clustered in the genome. Nucleic Acids Res. 1982 Oct 11;10(19):5905–5923. doi: 10.1093/nar/10.19.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman K. Antigenic variation in trypanosomes. Nature. 1978 Jun 22;273(5664):613–617. doi: 10.1038/273613a0. [DOI] [PubMed] [Google Scholar]
- Vickerman K. On the surface coat and flagellar adhesion in trypanosomes. J Cell Sci. 1969 Jul;5(1):163–193. doi: 10.1242/jcs.5.1.163. [DOI] [PubMed] [Google Scholar]


