Abstract
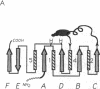
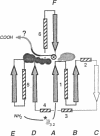
The bifunctional rat liver enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (ATP:D-fructose-6-phosphate 2-phosphotransferase/D-fructose-2,6-bisphosphate 2-phosphohydrolase, EC 2.7.1.105/EC 3.1.3.46) is constructed of two independent catalytic domains. We present evidence that the kinase and bisphosphatase halves of the bifunctional enzyme are, respectively, structurally similar to the glycolytic enzymes 6-phosphofructo-1-kinase and phosphoglycerate mutase. Computer-assisted modeling of the C-terminal bisphosphatase domain reveals a hydrophobic core and active site residue constellation equivalent to the yeast mutase structure; structural differences map to length-variable, surface-located loops. Sequence patterns derived from the structural alignment of mutases and the bisphosphatase further detect a significant similarity to a family of acid phosphatases. The N-terminal kinase domain, in turn, is predicted to form a nucleotide-binding fold that is analogous to a segment of 6-phosphofructo-1-kinase, suggesting that these unrelated enzymes bind fructose 6-phosphate and ATP substrates in a similar geometry. This analysis indicates that the bifunctional enzyme is the likely product of gene fusion of kinase and mutase/phosphatase catalytic units.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima K., Oshima T., Kubota I., Nakamura N., Mizunaga T., Toh-e A. The nucleotide sequence of the yeast PHO5 gene: a putative precursor of repressible acid phosphatase contains a signal peptide. Nucleic Acids Res. 1983 Mar 25;11(6):1657–1672. doi: 10.1093/nar/11.6.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa W., Meyhack B., Rudolph H., Schweingruber A. M., Hinnen A. Structural analysis of the two tandemly repeated acid phosphatase genes in yeast. Nucleic Acids Res. 1984 Oct 25;12(20):7721–7739. doi: 10.1093/nar/12.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F., Fletterick R. J. Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7872–7876. doi: 10.1073/pnas.85.21.7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Blouquit Y., Calvin M. C., Rosa R., Promé D., Promé J. C., Pratbernou F., Cohen-Solal M., Rosa J. Sequence of the human erythrocyte phosphoglycerate mutase by microsequencer and mass spectrometry. J Biol Chem. 1988 Nov 15;263(32):16906–16910. [PubMed] [Google Scholar]
- Brändeén C. I. Relation between structure and function of alpha/beta-proteins. Q Rev Biophys. 1980 Aug;13(3):317–338. doi: 10.1017/s0033583500001712. [DOI] [PubMed] [Google Scholar]
- El-Maghrabi M. R., Pate T. M., Murray K. J., Pilkis S. J. Differential effects of proteolysis and protein modification on the activities of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J Biol Chem. 1984 Nov 10;259(21):13096–13103. [PubMed] [Google Scholar]
- Elliott S., Chang C. W., Schweingruber M. E., Schaller J., Rickli E. E., Carbon J. Isolation and characterization of the structural gene for secreted acid phosphatase from Schizosaccharomyces pombe. J Biol Chem. 1986 Feb 25;261(6):2936–2941. [PubMed] [Google Scholar]
- Evans P. R., Hudson P. J. Structure and control of phosphofructokinase from Bacillus stearothermophilus. Nature. 1979 Jun 7;279(5713):500–504. doi: 10.1038/279500a0. [DOI] [PubMed] [Google Scholar]
- Fothergill L. A., Harkins R. N. The amino acid sequence of yeast phosphoglycerate mutase. Proc R Soc Lond B Biol Sci. 1982 Apr 22;215(1198):19–44. doi: 10.1098/rspb.1982.0026. [DOI] [PubMed] [Google Scholar]
- Gribskov M., McLachlan A. D., Eisenberg D. Profile analysis: detection of distantly related proteins. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4355–4358. doi: 10.1073/pnas.84.13.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joulin V., Peduzzi J., Roméo P. H., Rosa R., Valentin C., Dubart A., Lapeyre B., Blouquit Y., Garel M. C., Goossens M. Molecular cloning and sequencing of the human erythrocyte 2,3-bisphosphoglycerate mutase cDNA: revised amino acid sequence. EMBO J. 1986 Sep;5(9):2275–2283. doi: 10.1002/j.1460-2075.1986.tb04495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange A. J., Kummel L., el-Maghrabi M. R., Tauler A., Colosia A., Marker A., Pilkis S. J. Sequence of the 5'-flanking region of the rat 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase gene: regulation by glucocorticoids. Biochem Biophys Res Commun. 1989 Jul 31;162(2):753–760. doi: 10.1016/0006-291x(89)92374-7. [DOI] [PubMed] [Google Scholar]
- Le Boulch P., Joulin V., Garel M. C., Rosa J., Cohen-Solal M. Molecular cloning and nucleotide sequence of murine 2,3-bisphosphoglycerate mutase cDNA. Biochem Biophys Res Commun. 1988 Oct 31;156(2):874–881. doi: 10.1016/s0006-291x(88)80925-2. [DOI] [PubMed] [Google Scholar]
- Lee C. P., Kao M. C., French B. A., Putney S. D., Chang S. H. The rabbit muscle phosphofructokinase gene. Implications for protein structure, function, and tissue specificity. J Biol Chem. 1987 Mar 25;262(9):4195–4199. [PubMed] [Google Scholar]
- Lively M. O., el-Maghrabi M. R., Pilkis J., D'Angelo G., Colosia A. D., Ciavola J. A., Fraser B. A., Pilkis S. J. Complete amino acid sequence of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J Biol Chem. 1988 Jan 15;263(2):839–849. [PubMed] [Google Scholar]
- Murray K. J., El-Maghrabi M. R., Kountz P. D., Lukas T. J., Soderling T. R., Pilkis S. J. Amino acid sequence of the phosphorylation site of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J Biol Chem. 1984 Jun 25;259(12):7673–7681. [PubMed] [Google Scholar]
- Möller W., Amons R. Phosphate-binding sequences in nucleotide-binding proteins. FEBS Lett. 1985 Jul 1;186(1):1–7. doi: 10.1016/0014-5793(85)81326-0. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., Fox E., Wolfe L., Rothbarth L., Colosia A., Stewart H. B., el-Maghrabi M. R. Hormonal modulation of key hepatic regulatory enzymes in the gluconeogenic/glycolytic pathway. Ann N Y Acad Sci. 1986;478:1–19. doi: 10.1111/j.1749-6632.1986.tb15517.x. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., Lively M. O., el-Maghrabi M. R. Active site sequence of hepatic fructose-2,6-bisphosphatase. Homology in primary structure with phosphoglycerate mutase. J Biol Chem. 1987 Sep 15;262(26):12672–12675. [PubMed] [Google Scholar]
- Pilkis S. J., Regen D. M., Stewart H. B., Pilkis J., Pate T. M., El-Maghrabi M. R. Evidence for two catalytic sites on 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase. Dynamics of substrate exchange and phosphoryl enzyme formation. J Biol Chem. 1984 Jan 25;259(2):949–958. [PubMed] [Google Scholar]
- Pohlmann R., Krentler C., Schmidt B., Schröder W., Lorkowski G., Culley J., Mersmann G., Geier C., Waheed A., Gottschalk S. Human lysosomal acid phosphatase: cloning, expression and chromosomal assignment. EMBO J. 1988 Aug;7(8):2343–2350. doi: 10.1002/j.1460-2075.1988.tb03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder J. W., Richards F. M. Tertiary templates for proteins. Use of packing criteria in the enumeration of allowed sequences for different structural classes. J Mol Biol. 1987 Feb 20;193(4):775–791. doi: 10.1016/0022-2836(87)90358-5. [DOI] [PubMed] [Google Scholar]
- Rosa R., Blouquit Y., Calvin M. C., Prome D., Prome J. C., Rosa J. Isolation, characterization, and structure of a mutant 89 Arg----Cys bisphosphoglycerate mutase. Implication of the active site in the mutation. J Biol Chem. 1989 May 15;264(14):7837–7843. [PubMed] [Google Scholar]
- Rose Z. B. Intermediates in the phosphoglycerate mutase and bisphosphoglycerate synthase reactions. Methods Enzymol. 1982;87:42–51. doi: 10.1016/s0076-6879(82)87006-7. [DOI] [PubMed] [Google Scholar]
- Rose Z. B. The enzymology of 2,3-bisphosphoglycerate. Adv Enzymol Relat Areas Mol Biol. 1980;51:211–253. doi: 10.1002/9780470122969.ch5. [DOI] [PubMed] [Google Scholar]
- Sakoda S., Shanske S., DiMauro S., Schon E. A. Isolation of a cDNA encoding the B isozyme of human phosphoglycerate mutase (PGAM) and characterization of the PGAM gene family. J Biol Chem. 1988 Nov 15;263(32):16899–16905. [PubMed] [Google Scholar]
- Shanske S., Sakoda S., Hermodson M. A., DiMauro S., Schon E. A. Isolation of a cDNA encoding the muscle-specific subunit of human phosphoglycerate mutase. J Biol Chem. 1987 Oct 25;262(30):14612–14617. [PubMed] [Google Scholar]
- Shirakihara Y., Evans P. R. Crystal structure of the complex of phosphofructokinase from Escherichia coli with its reaction products. J Mol Biol. 1988 Dec 20;204(4):973–994. doi: 10.1016/0022-2836(88)90056-3. [DOI] [PubMed] [Google Scholar]
- Sowadski J. M., Handschumacher M. D., Murthy H. M., Foster B. A., Wyckoff H. W. Refined structure of alkaline phosphatase from Escherichia coli at 2.8 A resolution. J Mol Biol. 1985 Nov 20;186(2):417–433. doi: 10.1016/0022-2836(85)90115-9. [DOI] [PubMed] [Google Scholar]
- Starzyk R. M., Webster T. A., Schimmel P. Evidence for dispensable sequences inserted into a nucleotide fold. Science. 1987 Sep 25;237(4822):1614–1618. doi: 10.1126/science.3306924. [DOI] [PubMed] [Google Scholar]
- Summers N. L., Carlson W. D., Karplus M. Analysis of side-chain orientations in homologous proteins. J Mol Biol. 1987 Jul 5;196(1):175–198. doi: 10.1016/0022-2836(87)90520-1. [DOI] [PubMed] [Google Scholar]
- Tauler A., Lange A. J., el-Maghrabi M. R., Pilkis S. J. Expression of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and its kinase domain in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7316–7320. doi: 10.1073/pnas.86.19.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauler A., Rosenberg A. H., Colosia A., Studier F. W., Pilkis S. J. Expression of the bisphosphatase domain of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6642–6646. doi: 10.1073/pnas.85.18.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauler A., el-Maghrabi M. R., Pilkis S. J. Functional homology of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase, phosphoglycerate mutase, and 2,3-bisphosphoglycerate mutase. J Biol Chem. 1987 Dec 15;262(35):16808–16815. [PubMed] [Google Scholar]
- Taylor W. R. Pattern matching methods in protein sequence comparison and structure prediction. Protein Eng. 1988 Jul;2(2):77–86. doi: 10.1093/protein/2.2.77. [DOI] [PubMed] [Google Scholar]
- Touati E., Danchin A. The structure of the promoter and amino terminal region of the pH 2.5 acid phosphatase structural gene (appA) of E. coli: a negative control of transcription mediated by cyclic AMP. Biochimie. 1987 Mar;69(3):215–221. doi: 10.1016/0300-9084(87)90045-9. [DOI] [PubMed] [Google Scholar]
- Van Etten R. L. Human prostatic acid phosphatase: a histidine phosphatase. Ann N Y Acad Sci. 1982;390:27–51. doi: 10.1111/j.1749-6632.1982.tb40302.x. [DOI] [PubMed] [Google Scholar]
- Vihko P., Virkkunen P., Henttu P., Roiko K., Solin T., Huhtala M. L. Molecular cloning and sequence analysis of cDNA encoding human prostatic acid phosphatase. FEBS Lett. 1988 Aug 29;236(2):275–281. doi: 10.1016/0014-5793(88)80037-1. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Terpstra P., Hol W. G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986 Jan 5;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- Winn S. I., Watson H. C., Harkins R. N., Fothergill L. A. Structure and activity of phosphoglycerate mutase. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):121–130. doi: 10.1098/rstb.1981.0066. [DOI] [PubMed] [Google Scholar]
- Yanagawa S., Hitomi K., Sasaki R., Chiba H. Isolation and characterization of cDNA encoding rabbit reticulocyte 2,3-bisphosphoglycerate synthase. Gene. 1986;44(2-3):185–191. doi: 10.1016/0378-1119(86)90181-2. [DOI] [PubMed] [Google Scholar]
- el-Maghrabi M. R., Pate T. M., D'Angelo G., Correia J. J., Lively M. O., Pilkis S. J. Rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Identification of essential sulfhydryl residues in the primary sequence of the enzyme. J Biol Chem. 1987 Aug 25;262(24):11714–11720. [PubMed] [Google Scholar]