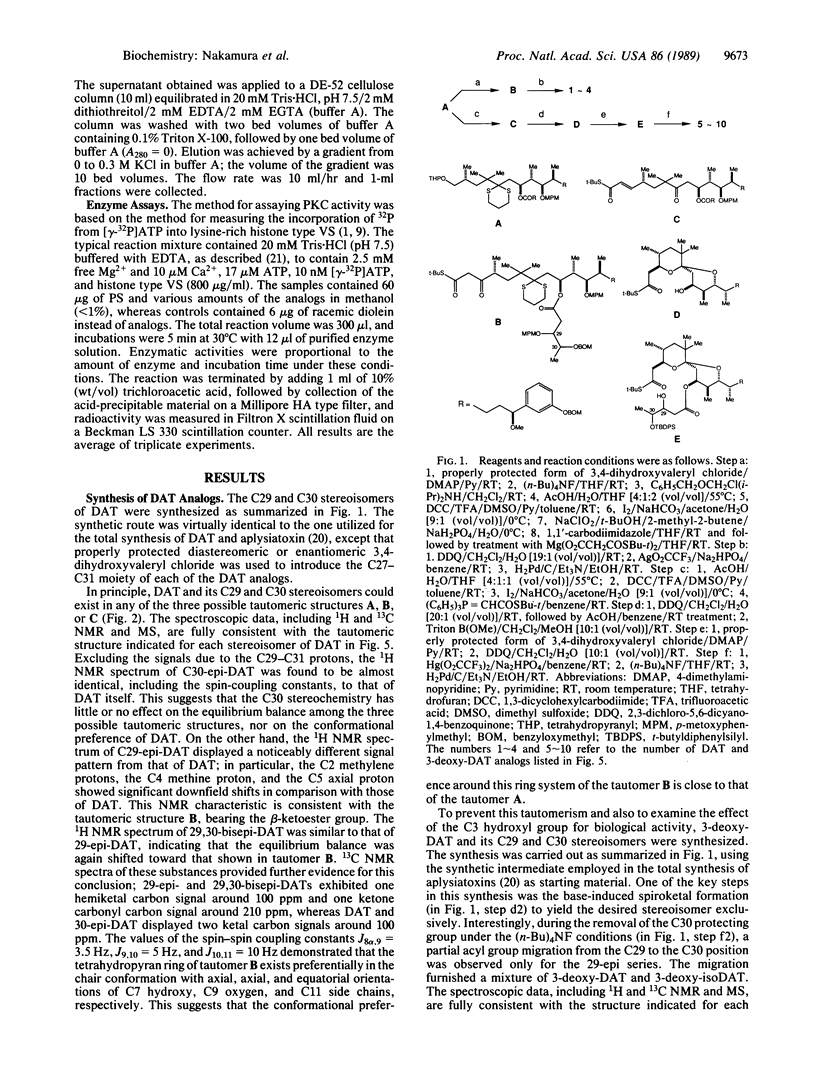
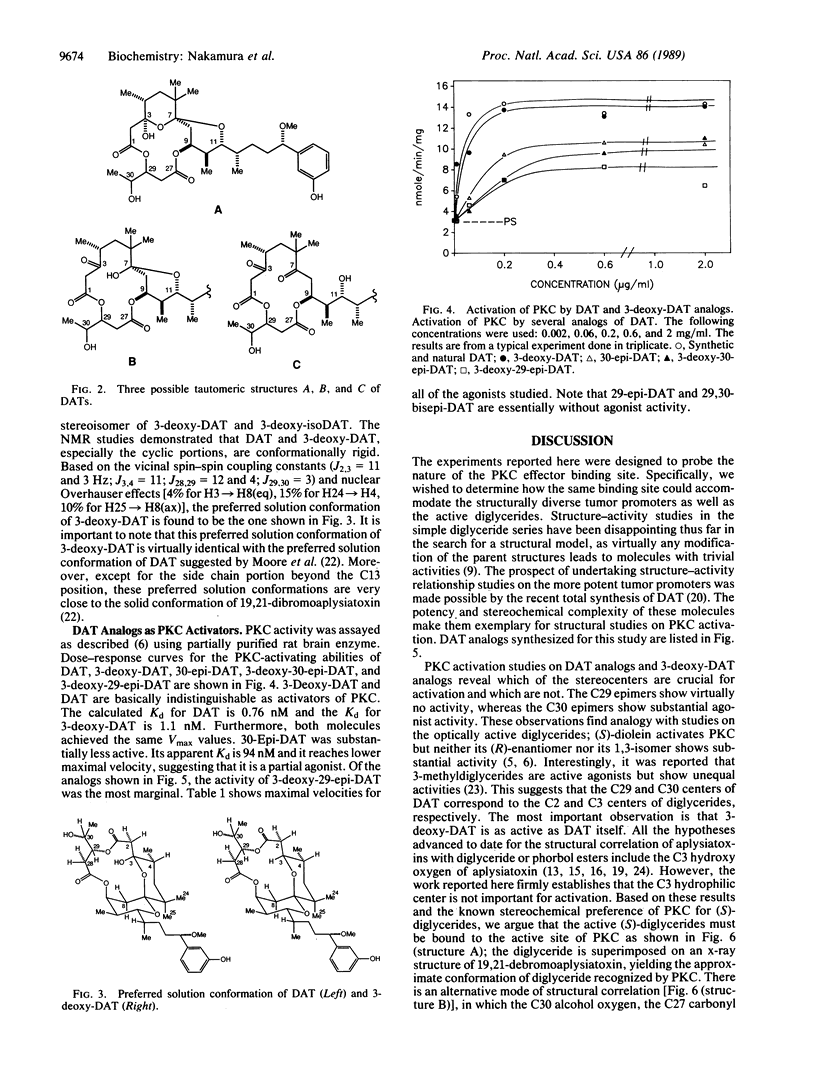
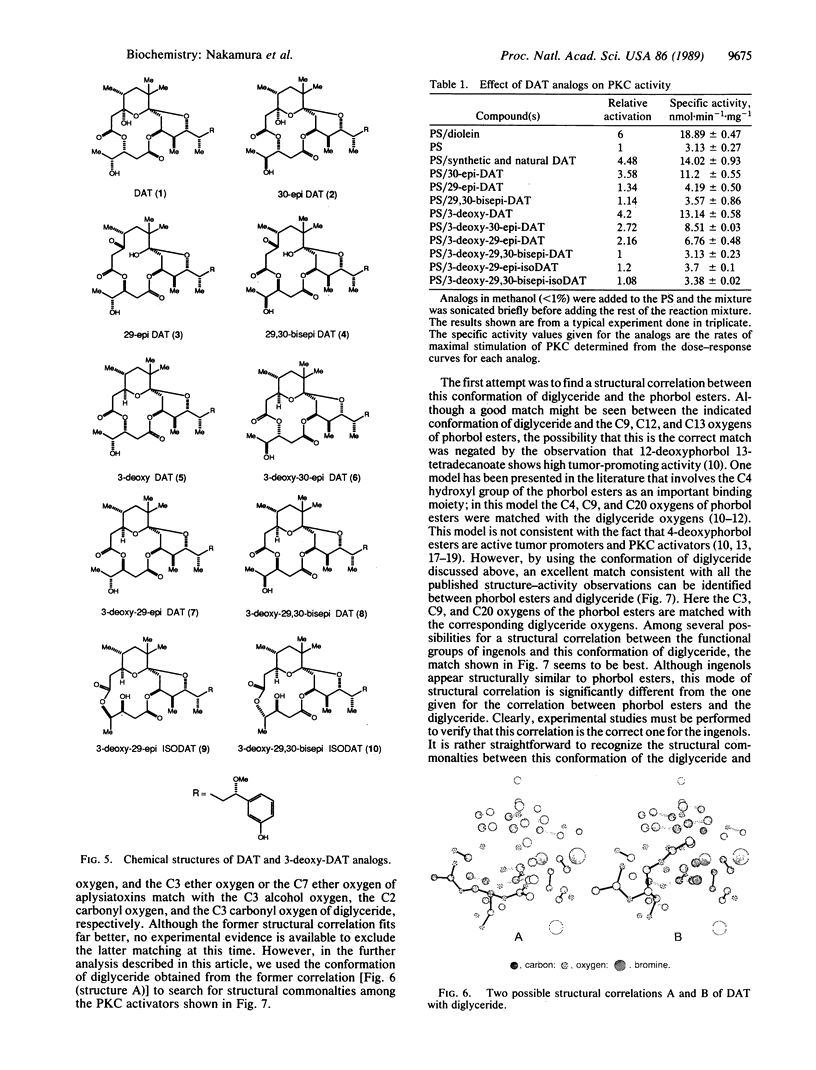
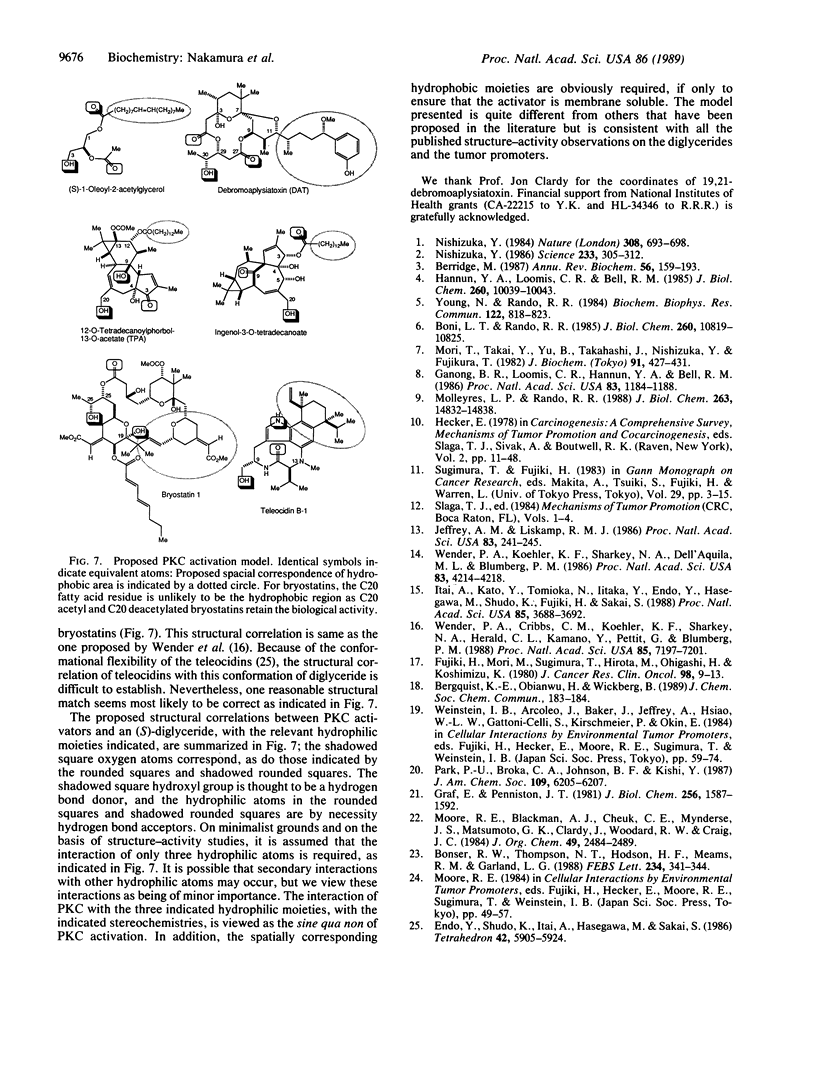
Abstract
Protein kinase C (PKC) is an important enzyme that helps govern cell metabolism and growth. The enzyme is physiologically activated when an (S)-diglyceride binds to its own regulatory domain. The saturable binding site of the regulatory domain can also be bound by any of a group of structurally diverse tumor promoters, including debromoaplysiatoxins (DATs), phorbol esters, ingenols, teleocidins, and bryostatins. The question of how the same binding site can be the target of these structurally diverse molecules is of considerable importance and is addressed in this article. The relatively rigid structure of DAT and the fact that it possesses a diglyceride moiety renders it an ideal starting template. Structure-activity studies with PKC reveal that the C29 but not the C30 stereocenter of DAT is critical for activity. Furthermore, 3-deoxy-DAT and DAT are equipotent as PKC activators, hence the C3 hydroxyl group of DAT is not critical for activity. Straightforward structural considerations show that the C30 hydroxyl group of DAT matches the C3 hydroxyl group of diglyceride, the C29 stereocenter of DAT matches the C2 stereocenter of (S)-diglyceride, and the C1 ester moiety of DAT matches the C2 ester moiety of diglyceride. Based on these studies and on published structure-activity observations on other tumor promoters, a structural hypothesis is developed to account for the chemical mechanism of tumor promoter action. Experimentally testable predictions are made concerning the interactions with PKC of several classes of tumor PKC activators.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Boni L. T., Rando R. R. The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J Biol Chem. 1985 Sep 5;260(19):10819–10825. [PubMed] [Google Scholar]
- Bonser R. W., Thompson N. T., Hodson H. F., Beams R. M., Garland L. G. Evidence that a second stereochemical centre in diacylglycerols defines interaction at the recognition site on protein kinase C. FEBS Lett. 1988 Jul 18;234(2):341–344. doi: 10.1016/0014-5793(88)80112-1. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Mori M., Sugimura T., Hirota M., Ohigashi H., Koshimizu K. Relationship between ornithine decarboxylase-inducing activity and configuration at C-4 in phorbol ester derivatives. J Cancer Res Clin Oncol. 1980;98(1):9–13. doi: 10.1007/BF00413172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganong B. R., Loomis C. R., Hannun Y. A., Bell R. M. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1184–1188. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf E., Penniston J. T. CaATP: the substrate, at low ATP concentrations, of Ca2+ ATPase from human erythrocyte membranes. J Biol Chem. 1981 Feb 25;256(4):1587–1592. [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Activation of protein kinase C by Triton X-100 mixed micelles containing diacylglycerol and phosphatidylserine. J Biol Chem. 1985 Aug 25;260(18):10039–10043. [PubMed] [Google Scholar]
- Itai A., Kato Y., Tomioka N., Iitaka Y., Endo Y., Hasegawa M., Shudo K., Fujiki H., Sakai S. A receptor model for tumor promoters: rational superposition of teleocidins and phorbol esters. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3688–3692. doi: 10.1073/pnas.85.11.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey A. M., Liskamp R. M. Computer-assisted molecular modeling of tumor promoters: rationale for the activity of phorbol esters, teleocidin B, and aplysiatoxin. Proc Natl Acad Sci U S A. 1986 Jan;83(2):241–245. doi: 10.1073/pnas.83.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleyres L. P., Rando R. R. Structural studies on the diglyceride-mediated activation of protein kinase C. J Biol Chem. 1988 Oct 15;263(29):14832–14838. [PubMed] [Google Scholar]
- Mori T., Takai Y., Yu B., Takahashi J., Nishizuka Y., Fujikura T. Specificity of the fatty acyl moieties of diacylglycerol for the activation of calcium-activated, phospholipid-dependent protein kinase. J Biochem. 1982 Feb;91(2):427–431. doi: 10.1093/oxfordjournals.jbchem.a133714. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Rando R. R., Young N. The stereospecific activation of protein kinase C. Biochem Biophys Res Commun. 1984 Jul 31;122(2):818–823. doi: 10.1016/s0006-291x(84)80107-2. [DOI] [PubMed] [Google Scholar]
- Wender P. A., Cribbs C. M., Koehler K. F., Sharkey N. A., Herald C. L., Kamano Y., Pettit G. R., Blumberg P. M. Modeling of the bryostatins to the phorbol ester pharmacophore on protein kinase C. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7197–7201. doi: 10.1073/pnas.85.19.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender P. A., Koehler K. F., Sharkey N. A., Dell'Aquila M. L., Blumberg P. M. Analysis of the phorbol ester pharmacophore on protein kinase C as a guide to the rational design of new classes of analogs. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4214–4218. doi: 10.1073/pnas.83.12.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]




