Abstract
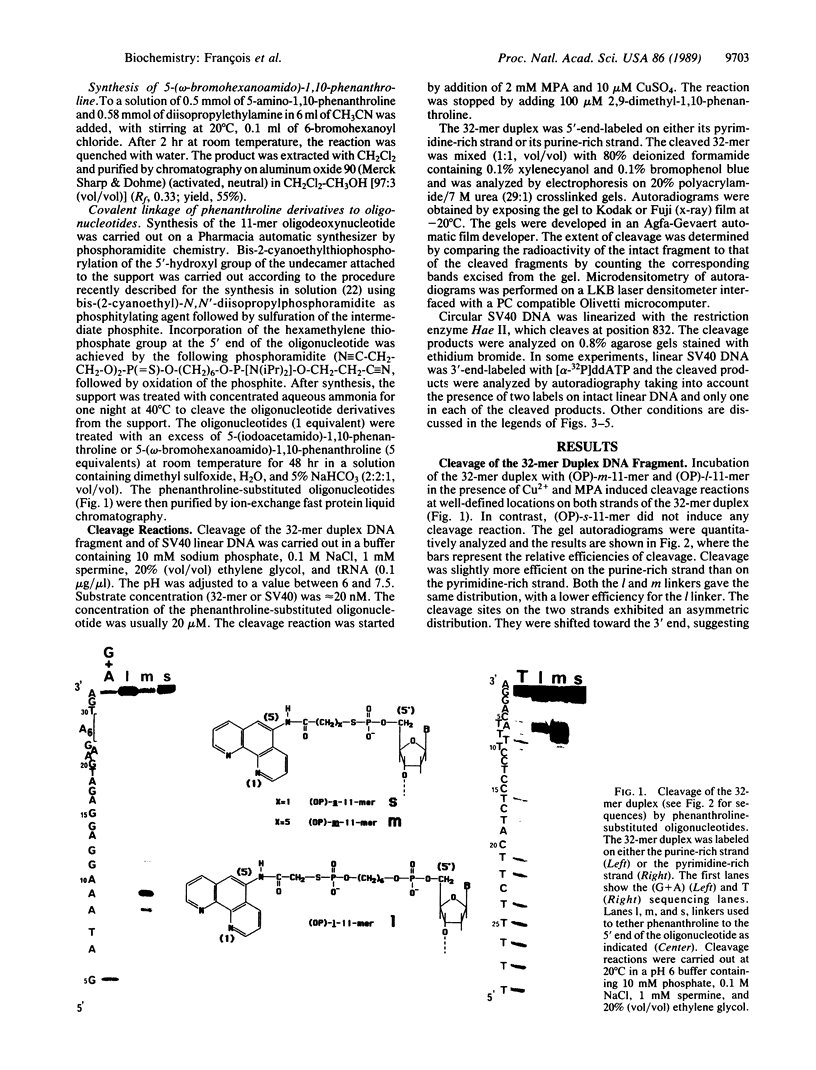
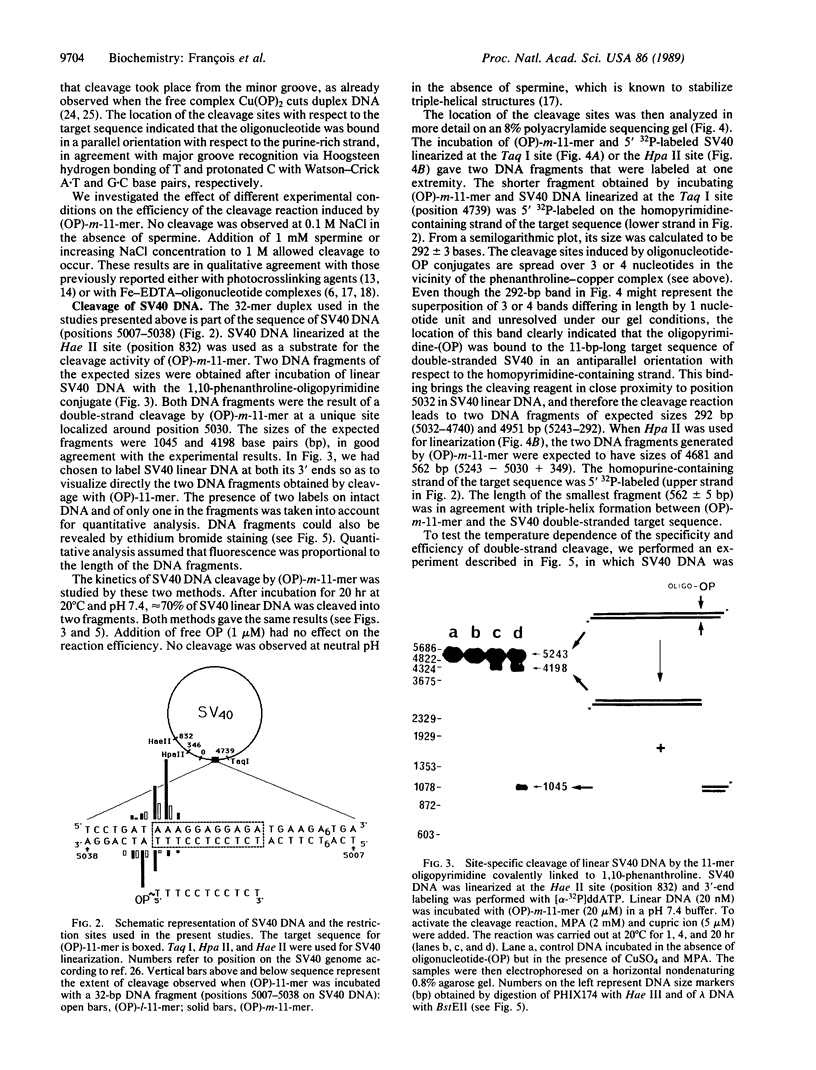
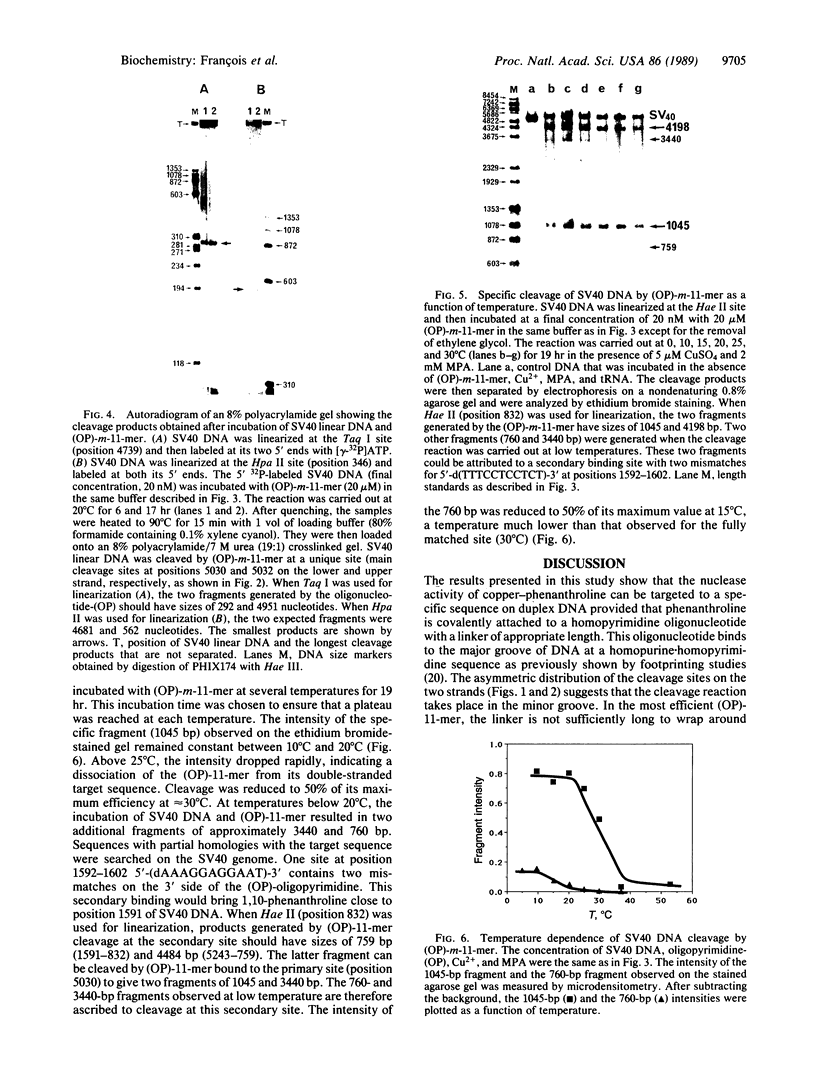
Homopyrimidine oligodeoxynucleotides recognize the major groove of the DNA double helix at homopurine.homopyrimidine sequences by forming local triple helices. Phenanthroline was covalently attached to the 5' end of an 11-mer homopyrimidine oligonucleotide of sequence d(TTTCCTCCTCT). Simian virus 40 DNA, which contains a single target site for this oligonucleotide, was used as a substrate for the phenanthroline-oligonucleotide conjugate. In the presence of copper ions and a reducing agent, a single specific double-strand cleavage site was observed at 20 degrees C by agarose gel electrophoresis. The efficiency of double-strand cleavage was greater than 70% at 20 degrees C and pH 7.4. Secondary cleavage sites were observed when binding of the oligonucleotide to mismatched sequences was allowed to take place at low temperature. The exact location of the cleavage sites was determined by polyacrylamide gel electrophoresis of denatured fragments by using both simian virus 40 DNA and a synthetic DNA fragment containing the target sequence. The asymmetric distribution of the cleavage sites on the two strands revealed that the cleavage reaction took place in the minor groove even though the phenanthroline linker was located in the major groove. Linkers of different lengths were used to tether phenanthroline to the oligonucleotide and their relative efficacies of DNA cleavage were compared. Based on these comparative studies and on model building, it is proposed that the phenanthroline ring carried by the oligonucleotide intercalates from the major groove and that copper chelation locks the complex in place from within the minor groove where the cleavage reaction occurs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boidot-Forget M., Chassignol M., Takasugi M., Thuong N. T., Hélène C. Site-specific cleavage of single-stranded and double-stranded DNA sequences by oligodeoxyribonucleotides covalently linked to an intercalating agent and an EDTA-Fe chelate. Gene. 1988 Dec 10;72(1-2):361–371. doi: 10.1016/0378-1119(88)90163-1. [DOI] [PubMed] [Google Scholar]
- Chen C. H., Sigman D. S. Nuclease activity of 1,10-phenanthroline-copper: sequence-specific targeting. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7147–7151. doi: 10.1073/pnas.83.19.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Nonenzymatic sequence-specific cleavage of single-stranded DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(4):963–967. doi: 10.1073/pnas.82.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer G. B., Dervan P. B. Sequence-specific cleavage of single-stranded DNA: oligodeoxynucleotide-EDTA X Fe(II). Proc Natl Acad Sci U S A. 1985 Feb;82(4):968–972. doi: 10.1073/pnas.82.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova O. S., Knorre D. G., Podust L. M., Zarytova V. F. Complementary addressed modification of double-stranded DNA within a ternary complex. FEBS Lett. 1988 Feb 15;228(2):273–276. doi: 10.1016/0014-5793(88)80014-0. [DOI] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Chassignol M., Thuong N. T., Helene C. Sequence-targeted cleavage of single- and double-stranded DNA by oligothymidylates covalently linked to 1,10-phenanthroline. J Biol Chem. 1989 Apr 5;264(10):5891–5898. [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Chassignol M., Thuong N. T., Sun J. S., Hélène C. Periodic cleavage of poly(dA) by oligothymidylates covalently linked to the 1,10-phenanthroline-copper complex. Biochemistry. 1988 Apr 5;27(7):2272–2276. doi: 10.1021/bi00407a004. [DOI] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Hélène C. Sequence-specific recognition of the major groove of DNA by oligodeoxynucleotides via triple helix formation. Footprinting studies. Nucleic Acids Res. 1988 Dec 23;16(24):11431–11440. doi: 10.1093/nar/16.24.11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorre D. G., Vlassov V. V. Complementary-addressed (sequence-specific) modification of nucleic acids. Prog Nucleic Acid Res Mol Biol. 1985;32:291–320. doi: 10.1016/s0079-6603(08)60352-9. [DOI] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Helene C., Chassignol M., Thuong N. T. Targeted cleavage of polynucleotides by complementary oligonucleotides covalently linked to iron-porphyrins. Biochemistry. 1986 Nov 4;25(22):6736–6739. doi: 10.1021/bi00370a002. [DOI] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Praseuth D., Habhoub N., Decout J. L., Thuong N. T., Lhomme J., Hélène C. Sequence-specific recognition, photocrosslinking and cleavage of the DNA double helix by an oligo-[alpha]-thymidylate covalently linked to an azidoproflavine derivative. Nucleic Acids Res. 1987 Oct 12;15(19):7749–7760. doi: 10.1093/nar/15.19.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. L., Murakami A., Blake K. R., Lin S. B., Miller P. S. Interaction of psoralen-derivatized oligodeoxyribonucleoside methylphosphonates with single-stranded DNA. Biochemistry. 1988 May 3;27(9):3197–3203. doi: 10.1021/bi00409a011. [DOI] [PubMed] [Google Scholar]
- Lyamichev V. I., Mirkin S. M., Frank-Kamenetskii M. D., Cantor C. R. A stable complex between homopyrimidine oligomers and the homologous regions of duplex DNAs. Nucleic Acids Res. 1988 Mar 25;16(5):2165–2178. doi: 10.1093/nar/16.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Neidle S., Abraham Z. Structural and sequence-dependent aspects of drug intercalation into nucleic acids. CRC Crit Rev Biochem. 1984;17(1):73–121. doi: 10.3109/10409238409110270. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Le Doan T., Chassignol M., Decout J. L., Habhoub N., Lhomme J., Thuong N. T., Hélène C. Sequence-targeted photosensitized reactions in nucleic acids by oligo-alpha-deoxynucleotides and oligo-beta-deoxynucleotides covalently linked to proflavin. Biochemistry. 1988 Apr 19;27(8):3031–3038. doi: 10.1021/bi00408a055. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Perrouault L., Le Doan T., Chassignol M., Thuong N., Hélène C. Sequence-specific binding and photocrosslinking of alpha and beta oligodeoxynucleotides to the major groove of DNA via triple-helix formation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1349–1353. doi: 10.1073/pnas.85.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trung Le Doan, Perrouault L., Chassignol M., Nguyen T. T., Hélène C. Sequence-targeted chemical modifications of nucleic acids by complementary oligonucleotides covalently linked to porphyrins. Nucleic Acids Res. 1987 Nov 11;15(21):8643–8659. doi: 10.1093/nar/15.21.8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal J. M., Rill R. L. Sequence specificity of DNA cleavage by bis(1,10-phenanthroline)copper(I): effects of single base pair transitions on the cleavage of preferred pyrimidine-purine-pyrimidine triplets. Biochemistry. 1989 Apr 18;28(8):3243–3250. doi: 10.1021/bi00434a019. [DOI] [PubMed] [Google Scholar]