Abstract
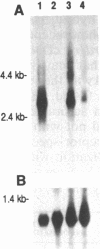
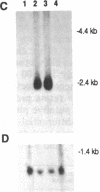
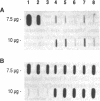
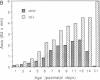
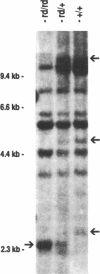
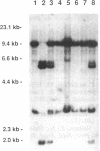
The inherited retinal degeneration of the rd mouse results in the exclusive loss of one cell type, the photoreceptors. We took advantage of this visual-cell loss to devise a strategy for the isolation of photoreceptor-specific cDNAs based on the use of subtractive and differential hybridizations. The resulting pool of photoreceptor-specific cDNAs was screened for a candidate cDNA for the rd gene, and a putative rd cDNA that maps to mouse chromosome 5, the chromosome to which the rd gene has been assigned, was identified. On Northern blots the candidate rd cDNA hybridizes a 3.3-kilobase RNA species from 9- to 11-day-old developing normal retina and, much more faintly, a 3.6-kb RNA species from age-matched rd retina. The 0.3-kilobase difference in the size of the mRNAs hybridized suggests that a structural alteration in the gene corresponding to the candidate rd cDNA has occurred in the rd mouse. This was further supported by the detection of polymorphisms between rd/rd and +/+ mouse genomic DNA after digestion with restriction endonucleases and probing with the candidate rd cDNA. Expression of mRNAs hybridized by the candidate rd cDNA is detected in normal and diseased retinas at postnatal day 1 but the signal intensity is considerably lower in the rd retina. To our knowledge, this is the earliest molecular defect reported in the rd retina that is observed prior to any phenotypic signs of photoreceptor degeneration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquirre G., Farber D., Lolley R., Fletcher R. T., Chader G. J. Rod-cone dysplasia in Irish setters: a defect in cyclic GMP metabolism in visual cells. Science. 1978 Sep 22;201(4361):1133–1134. doi: 10.1126/science.210508. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blanks J. C., Adinolfi A. M., Lolley R. N. Photoreceptor degeneration and synaptogenesis in retinal-degenerative (rd) mice. J Comp Neurol. 1974 Jul 1;156(1):95–106. doi: 10.1002/cne.901560108. [DOI] [PubMed] [Google Scholar]
- Bowes C., Farber D. B. mRNAs coding for proteins of the cGMP cascade in the degenerative retina of the rd mouse. Exp Eye Res. 1987 Oct;45(4):467–480. doi: 10.1016/s0014-4835(87)80058-1. [DOI] [PubMed] [Google Scholar]
- Bowes C., van Veen T., Farber D. B. Opsin, G-protein and 48-kDa protein in normal and rd mouse retinas: developmental expression of mRNAs and proteins and light/dark cycling of mRNAs. Exp Eye Res. 1988 Sep;47(3):369–390. doi: 10.1016/0014-4835(88)90049-8. [DOI] [PubMed] [Google Scholar]
- Caley D. W., Johnson C., Liebelt R. A. The postnatal development of the retina in the normal and rodless CBA mouse: a light and electron microscopic study. Am J Anat. 1972 Feb;133(2):179–212. doi: 10.1002/aja.1001330205. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson L. D., LaVail M. M., Sidman R. L. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978 Jun;17(6):489–498. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Danciger M., Kozak C. A., Farber D. B. The gene for the alpha-subunit of retinal rod transducin is on mouse chromosome 9. Genomics. 1989 Feb;4(2):215–217. doi: 10.1016/0888-7543(89)90303-0. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Cohen D. I., Nielsen E. A., Steinmetz M., Paul W. E., Hood L. Cell-type-specific cDNA probes and the murine I region: the localization and orientation of Ad alpha. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2194–2198. doi: 10.1073/pnas.81.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. B., Dawid I. B. Use of a cloned library for the study of abundant poly(A)+RNA during Xenopus laevis development. Dev Biol. 1980 May;76(2):449–464. doi: 10.1016/0012-1606(80)90393-0. [DOI] [PubMed] [Google Scholar]
- Farber D. B., Lolley R. N. Cyclic guanosine monophosphate: elevation in degenerating photoreceptor cells of the C3H mouse retina. Science. 1974 Nov 1;186(4162):449–451. doi: 10.1126/science.186.4162.449. [DOI] [PubMed] [Google Scholar]
- Farber D. B., Lolley R. N. Enzymic basis for cyclic GMP accumulation in degenerative photoreceptor cells of mouse retina. J Cyclic Nucleotide Res. 1976;2(3):139–148. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fodor E. J., Doty P. Highly specific transcription of globin sequences in isolated reticulocyte nuclei. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1478–1485. doi: 10.1016/s0006-291x(77)80145-9. [DOI] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hoggan M. D., Halden N. F., Buckler C. E., Kozak C. A. Genetic mapping of the mouse c-fms proto-oncogene to chromosome 18. J Virol. 1988 Mar;62(3):1055–1056. doi: 10.1128/jvi.62.3.1055-1056.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohne D. E., Levison S. A., Byers M. J. Room temperature method for increasing the rate of DNA reassociation by many thousandfold: the phenol emulsion reassociation technique. Biochemistry. 1977 Nov 29;16(24):5329–5341. doi: 10.1021/bi00643a026. [DOI] [PubMed] [Google Scholar]
- Koletsky A. J., Harding M. W., Handschumacher R. E. Cyclophilin: distribution and variant properties in normal and neoplastic tissues. J Immunol. 1986 Aug 1;137(3):1054–1059. [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of the ecotropic murine leukemia virus-inducing locus of BALB/c mouse to chromosome 5. Science. 1979 Apr 6;204(4388):69–71. doi: 10.1126/science.219475. [DOI] [PubMed] [Google Scholar]
- Kozak C., Nichols E., Ruddle F. H. Gene linkage analysis in the mouse by somatic cell hybridization: assignment of adenine phosphoribosyltransferase to chromosome 8 and alpha-galactosidase to the X chromosome. Somatic Cell Genet. 1975 Oct;1(4):371–382. doi: 10.1007/BF01538668. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Kim G. S., Prystowsky M. B., Lancki D. W., Sabath D. E., Pan J. L., Weissman S. M. Isolation and initial characterization of multiple species of T-lymphocyte subset cDNA clones. Proc Natl Acad Sci U S A. 1987 May;84(9):2896–2900. doi: 10.1073/pnas.84.9.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASANSKY A., DE ROBERTIS E. Submicroscopic analysis of the genetic distrophy of visual cells in C3H mice. J Biophys Biochem Cytol. 1960 Jul;7:679–684. doi: 10.1083/jcb.7.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail M. M., Mullen R. J. Role of the pigment epithelium in inherited retinal degeneration analyzed with experimental mouse chimeras. Exp Eye Res. 1976 Aug;23(2):227–245. doi: 10.1016/0014-4835(76)90206-2. [DOI] [PubMed] [Google Scholar]
- LaVail M. M., Sidman R. L. C57BL-6J mice with inherited retinal degeneration. Arch Ophthalmol. 1974 May;91(5):394–400. doi: 10.1001/archopht.1974.03900060406015. [DOI] [PubMed] [Google Scholar]
- Lolley R. N., Farber D. B., Rayborn M. E., Hollyfield J. G. Cyclic GMP accumulation causes degeneration of photoreceptor cells: simulation of an inherited disease. Science. 1977 May 6;196(4290):664–666. doi: 10.1126/science.193183. [DOI] [PubMed] [Google Scholar]
- NOELL W. K. Studies on visual cell viability and differentiation. Ann N Y Acad Sci. 1959 Nov 12;74(2):337–361. doi: 10.1111/j.1749-6632.1958.tb39556.x. [DOI] [PubMed] [Google Scholar]
- Rigas B., Welcher A. A., Ward D. C., Weissman S. M. Rapid plasmid library screening using RecA-coated biotinylated probes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9591–9595. doi: 10.1073/pnas.83.24.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIDMAN R. L., GREEN M. C. RETINAL DEGENERATION IN THE MOUSE: LOCATION OF THE RD LOCUS IN LINKAGE GROUP XVII. J Hered. 1965 Jan-Feb;56:23–29. doi: 10.1093/oxfordjournals.jhered.a107364. [DOI] [PubMed] [Google Scholar]
- Sonohara O., Shiose Y. [Electron microscopic study of the visual cell of inherited retinal dystrophic mice]. Nihon Ganka Kiyo. 1968 Jan;19(1):77–86. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E. Developmental gene regulation in Aspergillus nidulans. Dev Biol. 1980 Aug;78(2):497–510. doi: 10.1016/0012-1606(80)90349-8. [DOI] [PubMed] [Google Scholar]
- Travis G. H., Sutcliffe J. G. Phenol emulsion-enhanced DNA-driven subtractive cDNA cloning: isolation of low-abundance monkey cortex-specific mRNAs. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1696–1700. doi: 10.1073/pnas.85.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford B. J., Liu Y., Fletcher R. T., Chader G. J., Farber D. B., Santos-Anderson R., Tso M. O. Cyclic nucleotide metabolism in inherited retinopathy in collies: a biochemical and histochemical study. Exp Eye Res. 1982 May;34(5):703–714. doi: 10.1016/s0014-4835(82)80031-6. [DOI] [PubMed] [Google Scholar]