Abstract
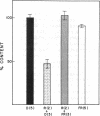
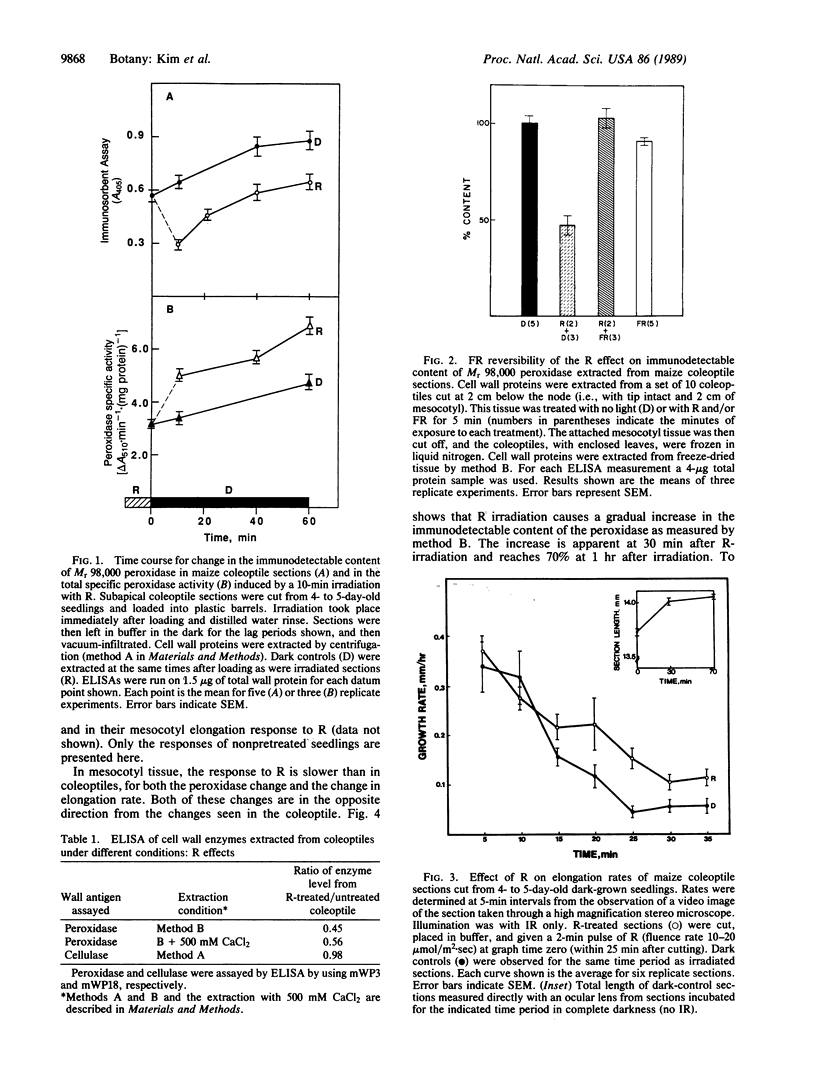
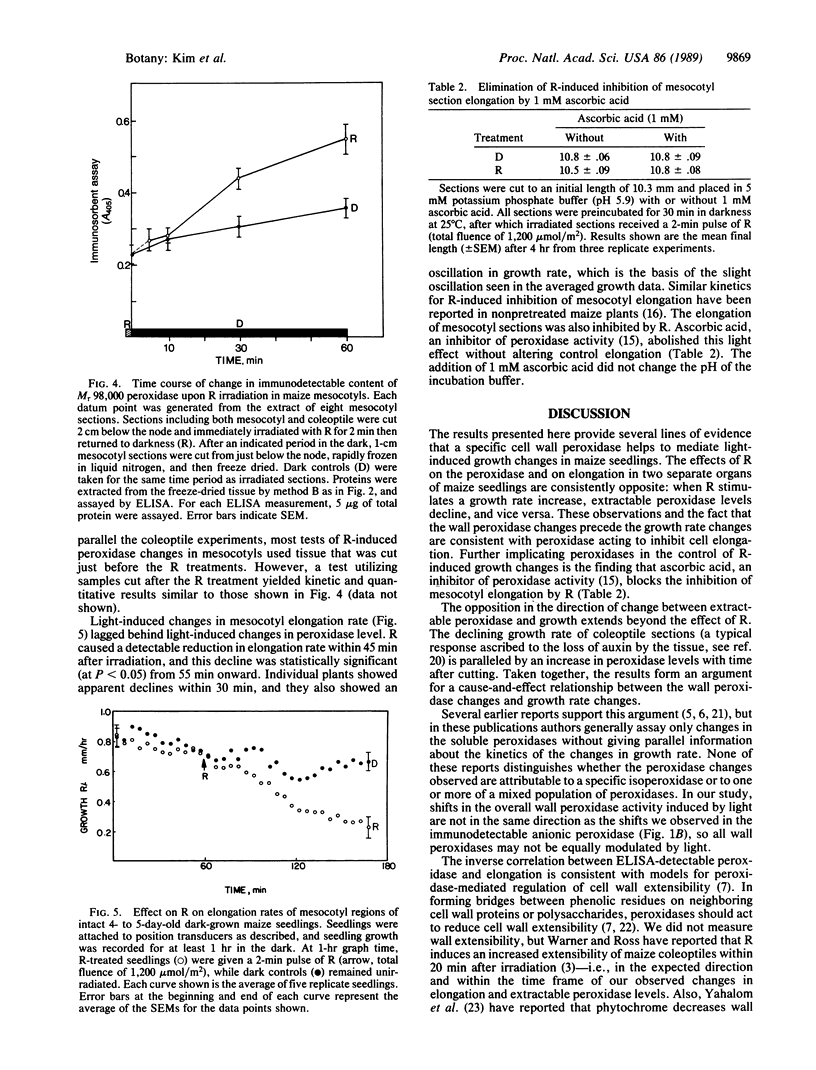
The regulatory pigment phytochrome induces rapid and opposite growth changes in different regions of etiolated maize seedling: it stimulates the elongation rate of coleoptiles and inhibits that of mesocotyls. As measured by a quantitative immunoassay, phytochrome also promotes rapid and opposite changes in the extractable content of a Mr 98,000 anionic isoperoxidase in the cell walls of these same organs: it induces a decrease of this peroxidase in coleoptiles and an increase in mesocotyls. The peroxidase changes precede the growth changes. As measured by video stereomicroscopy or a position transducer, red light (R), which photoactivates phytochrome, stimulates coleoptile elongation with a lag of about 15-20 min and suppresses mesocotyl growth with a lag of 45-50 min. R also induces a 50% reduction in the extractable level of the anionic peroxidase in coleoptile walls in less than 10 min and a 40% increase in the level of this peroxidase in mesocotyl walls within 30 min. Ascorbic acid, an inhibitor of peroxidase activity, blocks the effects of R on mesocotyl section growth. These results are relevant to hypotheses that postulate that certain wall peroxidases can participate in light-induced changes in growth rate by their effects on wall extensibility.
Keywords: ELISA, monoclonal antibody, wall enzyme, wall extensibility, Zea
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. R., Roux S. J. Characterization of nucleoside triphosphatase activity in isolated pea nuclei and its photoreversible regulation by light. Plant Physiol. 1986;81:609–613. doi: 10.1104/pp.81.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E. Reevaluation of the Effect of Calcium Ions on Auxin-induced Elongation. Plant Physiol. 1977 Nov;60(5):709–712. doi: 10.1104/pp.60.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J. Characterization of long-term extension of isolated cell walls from growing cucumber hypocotyls. Planta. 1989;177:121–130. [PubMed] [Google Scholar]
- Everdeen D. S., Kiefer S., Willard J. J., Muldoon E. P., Dey P. M., Li X. B., Lamport D. T. Enzymic cross-linkage of monomeric extensin precursors in vitro. Plant Physiol. 1988 Jul;87(3):616–621. doi: 10.1104/pp.87.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale C. C., Roux S. J. Photoreversible calcium fluxes induced by phytochrome in oat coleoptile cells. Plant Physiol. 1980 Apr;65(4):658–662. doi: 10.1104/pp.65.4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Rapid Auxin-induced Decrease in Free Space pH and Its Relationship to Auxin-induced Growth in Maize and Pea. Plant Physiol. 1976 Aug;58(2):203–209. doi: 10.1104/pp.58.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Terry M. E., Hoops P., Dauwalder M., Roux S. J. Production and characterization of monoclonal antibodies to wall-localized peroxidases from corn seedlings. Plant Physiol. 1988;88:1446–1453. doi: 10.1104/pp.88.4.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nelson A. J., Evans M. L. Analysis of growth patterns during gravitropic curvature in roots of Zea mays by use of a computer-based video digitizer. J Plant Growth Regul. 1986;5:73–83. doi: 10.1007/BF02025958. [DOI] [PubMed] [Google Scholar]
- Pike C. S., Richardson A. E. Phytochrome-controlled Hydrogen Ion Excretion by Avena Coleoptiles. Plant Physiol. 1977 Apr;59(4):615–617. doi: 10.1104/pp.59.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkle J. R., Jones R. L. Inhibition of stem elongation in cucumis seedlings by blue light requires calcium. Plant Physiol. 1988 Mar;86(3):960–966. doi: 10.1104/pp.86.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkle J. R. Photobiology of phytochrome-mediated growth responses in sections of stem tissue from etiolated oats and corn. Plant Physiol. 1986 Jun;81(2):533–537. doi: 10.1104/pp.81.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Quail P. H., Briggs W. R. Red Light-inhibited Mesocotyl Elongation in Maize Seedlings: II. Kinetic and Spectral Studies. Plant Physiol. 1979 Jun;63(6):1062–1067. doi: 10.1104/pp.63.6.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper M. J., Evans M. L. Time-dependent Changes in the Auxin Sensitivity of Coleoptile Segments: Apparent Sensory Adaptation. Plant Physiol. 1978 Feb;61(2):204–208. doi: 10.1104/pp.61.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner T. J., Ross J. D. Phytochrome control of maize coleoptile section elongation: the role of cell wall extensibility. Plant Physiol. 1981 Nov;68(5):1024–1026. doi: 10.1104/pp.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]