Abstract
Our study on long-term outcome of presymptomatic testing for Huntington disease had two aims: the comparison of the psychological well-being and social adjustment of carriers and non-carriers of the mutation, and the identification of psychological determinants to improve care/support of testees. We performed a cross-sectional study of 351 persons who underwent presymptomatic testing. Those who had motor signs were excluded from the comparison of asymptomatic carrier and non-carriers. A structured interview including five self-report scales and the MINI (Mini International Neuropsychiatric Inventory) was proposed to detect a psychopathology or problem with social adjustment.
We interviewed 119 testees (53%), 62 non-carriers and 57 carriers after a mean delay of 3.7 years (range: 0.32 to 8.9) after their result. Depression was frequent in asymptomatic carriers (58%). Interestingly, the self reported impact of the test showed that 27% of non-carriers did not cope well with a favourable result, and a significant percentage of non-carriers (24%) were depressed during follow-up. Multivariate analysis showed that only a previous episode of depression was predictive of depression after genetic testing in both carriers and non-carriers of the HD mutation (P<0.0001).
Psychological support is necessary for all testees regardless of the result of their presymptomatic test, because psychiatric care is often needed by both carriers and non-carriers.
Keywords: presymptomatic testing, Huntington disease, depression
Introduction
Identification of the molecular basis of many inherited disorders makes presymptomatic testing (PT) possible. PT reveals the genetic status of a person at risk without predicting the age at which the disease will become apparent or its severity. The counselling procedure developed for PT in Huntington disease (HD), an autosomal dominant late-onset neurodegenerative disorder with progressive behavioural, cognitive abnormalities and chorea, has become a model of how to proceed.
It has been offered since 1993 within a multidisciplinary framework,1 including a geneticist, a psychologist, a social worker, and nurse specialized in genetics, before and after blood sampling and testing. This is mandatory since the result will be definitive and the person will not be able to ignore it. Persons at risk for HD are faced with a Shakespearian question: To know or not to know? Do I want to know my status? Will I cope better with the certainty that I will develop the disease or with the uncertainty attached to ignorance of my genetic status? The team should support the decision of the testee, without interfering in his choice, and remain neutral.
In Europe, less than 20% of the at-risk population takes the test.2, 3, 4, 5 Previous studies have provided data on the socio-demographic characteristics, psychological profiles and motivations of the testees.4, 6, 7, 8, 9, 10, 11, 12 Other studies have focused on the psychosocial impact on individuals of PT for HD. They have shown that major catastrophic events, such as suicide do not occur more frequently after PT than in the general population.2, 8, 11, 13 They showed that depression and anxiety levels in carriers are similar before and 1 year after obtaining the test result, although confirmed carriers have higher scores on the Beck Hopelessness Scale.9 It was shown that depression and anxiety significantly decreased 5-year post test. Interestingly, this evolution was independent of the genetic result.8 A higher psychological distress 5-year post test was significantly associated with lower ego strength and unspecified motivation in the pre test period.8 This study was undertaken to compare the psychological well-being and social adjustment of carriers and non-carriers of the mutation after a long-term follow-up (up to 9 years), and to identify factors playing part in post test distress.
Subjects and methods
Between 1992 and 2001, 748 persons at risk for HD asked for PT at the genetics outpatient clinic at the Salpêtrière University Hospital (Paris, France) and initiated the testing procedure. This includes genetic counselling as well as psychological and social interviews before blood sampling to determine the person's subjective perception of risk and his motivation for testing. After a variable delay and multi-step counselling, 351 (47%) decided to have the test performed and obtained their result. Molecular analyses were performed as described.14 All testees were offered long-term follow up as part of the testing procedure. All (n=351) had given permission to be re-approached and were offered by mail an interview with a psychiatrist (SL), which was held at our institution. To preserve confidentiality as much as possible, the letter bore no indication that it came from the hospital or concerned PT, and no other means of communication was used to contact the testees. If there was no response within 6 months, a second letter was sent.
All carriers considered themselves as being unaffected. However, observation of undoubtful signs such as chorea or other extrapyramidal signs, that is, dystonic posture or bradykinesia, indicated that a carrier was clinically affected. The unambiguously affected carriers (n=18) were not included in the analysis in order to allow an unbiased comparison between carriers and non-carriers. The interview was designed to assess:
(a) life-events, their date and their subjective impact, ranked from 0 to 100 for each event (Amiel–Lebigre hetero-questionnaire),15 (b) psychiatric treatments or follow-up; (c) the efficacy of the coping strategies to cope with the result of the test. The latter was evaluated by the response to the question: ‘How do you feel about your result?' The answers were categorized according to the reaction strategies that were used to reduce the level of stress linked to the genetic test result. Coping was considered not efficient, if it did not reduce the impact of the event, and in this case, stress and anxiety were invoked. This could include extreme reactions such as the development of obsessive compulsive disorder, self-observation, somatic preoccupations or feeling affected by the disease and so forth. In contrast, coping was considered as being efficient if the strategies decreased the impact of the result (feeling of well-being, seeking social support, adapting to the new genetic status and so on);16 (d) Depression was evaluated with the Beck Depression Inventory (short version),17 followed by the MINI (Mini International Neuropsychiatric Inventory),18 to confirm the diagnosis of current depression if the Beck depression score is ≥4 (mild depression); (e) hopelessness was assessed by Beck Hopelessness Scale (BHS).19 Information about episodes of depression before the test or since the test was collected during the psychiatric interview. Anxiety was evaluated with the State and Trait Anxiety Inventory of Spielberger (STAI I and II). It evaluates independently the actual anxiety at the moment of the assessment (anxiety state) and the more general and long-standing quality of ‘trait anxiety' (anxiety-trait)20; (e) A scale of subjective distress, the Impact of Event Scale,21 was used to evaluate the impact of the test result (avoidance or intrusive thinking), The Social Adjustment Scale (SAS)22 was used to assess subjective adaptation in work, social life, leisure, family, matrimonial and home relationships and material resources.
Statistical analysis
Comparisons between groups were performed with Student's t-test for continuous variables and the χ2 or Fisher exact tests for categorical variables.
We used univariate and multivariate analysis to identify factors, which could be involved in the occurrence of depression after the result, such as: carrier versus non-carriers, sex, age at the time of the result, delay between first contact and result, marital status (single or divorced versus others), having children (yes/no), history of depression, sex of the affected parent, time of being aware of the genetic risk (>5 years versus other), test motivation (motivations such as desire to know or preparing the future versus for the offspring or parental project). As for all other comparisons, we excluded symptomatic carriers from the analyses. Owing to different delays between the result and study interview, we used a parametric model for censored data. Univariate predictors with P<0.05 were included in the stepwise multivariable model. The alpha level was set at 0.05. All analyses were performed with the SAS software version 8.2 (SAS Institute, Cary, NC, USA).
Results
Responders and non-responders
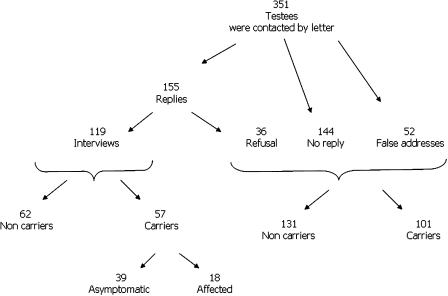
We were able to contact 299 out of the 351 testees, 52 letters were returned because of a wrong address (Figure 1). One hundred and fifty-five subjects (53%, n=155/299) responded to the proposal and 119 (40%, n=119/299) accepted the proposed interview, including 62 non-carriers and 57 carriers (see figure). The median duration of presymptomatic testing procedure, that is, the time between the first contact and the result, was 5.7 months (0.9–69.3) in carriers versus 5.2 months (0.83—38.7) in non-carriers. To ensure that there was no bias for further interpretation of the results, the 155 responders and the 144 non-responders were compared. The proportion of carriers versus non-carriers among responders and non-responders were similar (68/87 and 65/79, P=0.96). There were more women than men in both groups (66 and 59%, P=0.18). The sex of the transmitting parent was equally distributed; fathers represented 48 versus 47% in both groups (P=0.82). However, the responders were slightly older (36.37±10.53 versus 33.36±9.53, P=0.006) and the delay between obtaining of the result and the proposed interview was shorter (3.8±2.5 versus 4.6±2.6, P=0.003). Non-responders and responders lived within easy travel distance from our institution (63 versus 62%).
Figure 1.
Schema of this study including 351 individuals at risk for Huntington disease who underwent presymptomatic testing and 119 who accepted to undergo an interview and examination after they obtained a result.
Interviewed testees
The mean age of the responders when interviewed was 41.9±10.6 years (range: 21–66) (Table 1). The median delay between obtaining the test result and the interview, which was as long as 9 years, was 3.7 years. It was similar in carriers and non-carriers (3.7 versus 3.5 years). Carriers had a mean CAG repeat size of 42.6±2.9 (38–54). The disease was inherited from the mother in 54%, as expected for an autosomal dominant disease. Neurological examination showed that 18/57 carriers were already affected. We excluded them from further comparisons, leaving 39 asymptomatic carriers.
Table 1. Clinical characteristics of 119 testees interviewed after presymptomatic testing for Huntington disease.
| All persons | Non carriers n=62 | Carriers n=57 | ||
|---|---|---|---|---|
| n=119 | n=62 | Asymptomatic carriers n=39 | Symptomatic carriers n=18 | |
| Women/men (%) | 62:38 | 66:34 | 62:38 | 50:50 |
| Age at interview (mean±SD, range) | 41.9±10.6 (21.3–65.7) | 41.97±10.81 (21.3–61.4) | 40.5±10.5 (22.2–65.7) | 44.7±10.1 (24.6–63.2) |
| Age at first contact before testing (mean±SD, range) | 37.5±10.4 (18.1–62.6) | 37.65±10.41 (18.1–58.4) | 36.3±10.6 (19.5–62.6) | 39.3±10.3 (22.3–58.7) |
| Delay in months between 1st contact and result (median and range) | 5.4 (0.8 – 69.3) | 5.2 (0.8–38.7) | 5.4 (1.2–28) | 7.6 (0.93–69.3) |
| Delay in years after result and interview in years (median, range) | 3.7 (0.3 – 8.4) | 3.5 (0.3–8.9) | 3.7 (0.3–8.4) | 3.5 (0.4–7.9) |
| Maternal: paternal inheritance (%) | 53:47 | 41:59 | 67:33 | 67:33 |
Social adjustment after the test
The overall scores for social adjustment were similar in asymptomatic carriers and non-carriers, and were in the normal range for both groups (Table 2).
Table 2. Social adjustment of asymptomatic carriers and non-carriers after presymptomatic testing for Huntington disease.
| Non-carriers n=62 | Asymptomatic carriers n=39 | P | |
|---|---|---|---|
| Psychosocial adjustment total score (mean±SD)a | 1.58±0.32 | 1.63±0.38 | 0.5 |
| Work score (mean±SD) | 1.34±0.31 | 1.49±0.52 | 0.09 |
| Social life score (mean±SD) | 1.84±0.45 | 1.79±0.59 | 0.6 |
| Family relationships score (mean±SD) | 1.54±0.48 | 1.53±0.38 | 0.8 |
| Home score (mean±SD) | 1.55±0.48 | 1.68±0.54 | 0.2 |
| Resources score (mean±SD) | 1.29±0.66 | 1.28±0.76 | 0.9 |
1 and 2: normal, 3: mild difficulties, 4: moderate 5: pronounced 6 and 7: severe.
Psychiatric adjustment after the test
Carriers were not more anxious than non-carriers (Table 3). However, current depression was significantly more frequent in the former than in the latter (58 versus 24%, P=0.05) (Table 3). The same percentage of carriers and non-carriers had experienced depressive episodes before PT (42 versus 45%, P=0.8). After PT, the percentage of carriers experiencing depression increased from 42 to 49%, whereas the percentage of non-carriers decreased from 45 to 31%. It is nonetheless instructive that 31% of non-carriers remained depressed.
Table 3. Psychiatric profile of asymptomatic carriers and non carriers after presymptomatic testing for Huntington disease.
| Non-carriers n=62 | Asymptomatic carriers n=39 | P | |
|---|---|---|---|
| State anxiety (mean±SD, range)a | 32.83±8.92 (20–60) | 34.29±10.93 (20–63) | 0.47 |
| Trait anxiety (mean±SD)a | 37.60±8.81 (23–59) | 39.68±11.38 (24–74) | 0.31 |
| Current depression assessed by MINI (%) | 24 | 58 | 0.05 |
| Subjects with depression before PT (%) | 45 | 42 | 0.8 |
| Subjects with depression after PT (%) | 31 | 49 | 0.06 |
| Current depression score (mean,±SD, range) Beck scoreb | 2.89±3.03 (0–11) | 3.51±5.74 (0–24) | 0.5 |
| Current hopelessness Beck score (mean±SD, range)b | 3.21±2.33 (0–10) | 5.57±4.43 (0–15) | 0.002 |
| Retrospective impact score (0–100) of the test result (mean±SD) | 80.4±27.3 (0–100) | 72.0±28.9 (0–100) | 0.2 |
| Actual Impact Event Scale (maximal negative value with the result=45) | 8.15±7.99 (0–32) | 12.66±8.36 (0–34) | 0.008 |
| No change in coping with the result, efficient coping with the result and inefficient coping with the result | 21% 52% 27% | 18% 18% 64% | 0.0006 |
| Current psychiatric care (%) | 14.5 | 31 | 0.05 |
| Current treatment with antidepressive or anxiolytic substances (%) | 15 | 36 | 0.01 |
| Number of suicide attempts (n) | 3 | 1 | ns |
State anxiety scores: >65 very high anxiety level, 56–65 high anxiety level, 46–55 moderate anxiety level, 36–45 mild anxiety level, <36 very mild anxiety level.
Beck scores: 0–4 no depression, 4–7 mild depression, 8–15 moderate depression, >16 severe depression (max value 39).
Although the intensity of current depression assessed by the Beck Inventory Scale was similar in both groups, carriers had significantly higher scores than non-carriers when depression was evaluated with the Hopelessness Scale (5.57±4.43 versus 3.21±2.33, P=0.002). There was one suicide attempt and one hospitalization in the psychiatric department for major depression after PT in carriers. It is important to note, however, that three non-carriers also attempted suicide, one was hospitalized for depression and one for a psychotic episode. Inspite of this evident distress, however, only 31% of the carriers and 15% of non-carriers were under psychiatric care, a difference that was statistically significant (P=0.05); similarly only 36% of the carriers and 15% of the non-carriers were under treatment with antidepressive or anxiolytic drugs (P=0.01).
Univariate analysis aiming to identify factors in relation to depression after the result showed significant values for (i) being carrier (P=0.007); (ii) having as test motivation ‘other than for the offspring' (P=0.02) and (iii) having a history of depression before the test (P<0.0001) (Table 4). Multivariate analysis showed that the only predictive factor for the occurrence of depression after the test was the presence of a previous depressive episode (P<0.0001). Interestingly, among persons who experienced at least one depressive episode since the result, 88% of the non-carriers and 75% of carriers had already been depressed before taking the test. In individuals without depression before PT, only 6% of the non-carriers and 19% of the carriers became depressed (P=0.2).
Table 4. Univariate analysis of factors predictive for depression after the test result.
| Factors | Relative risk (95% CI) | P |
|---|---|---|
| Sex (female) | 1.20 (0.60–2.38) | 0.60 |
| Sex of the affected parent (female) | 1.14 (0.60–2.17) | 0.69 |
| Marital status (single or divorced versus others) | 1.45 (0.73–2.87) | 0.29 |
| Having children | 1.13 (0.57–2.24) | 0.74 |
| History of depression | 10.8 (4.39–26.61) | <0.0001 |
| Time of being aware of the genetic risk (>5 years versus others) | 1.39 (0.73–2.66) | 0.32 |
| Motivation to do test (other than ‘for the offspring or parental project') | 2.56 (1.12–5.83) | 0.02 |
| Delay between first contact and the result | 1.07 (0.97–1.18) | 0.21 |
| Age at the time of the result | 1.00 (0.99–1.01) | 0.12 |
| Carriers versus non carriers | 1.85 (0.97–3.52) | 0.06 |
When asked to retrospectively rate the impact of their test result on their lives on a scale of 100, both carriers and non-carriers gave similarly high scores (Table 3). However, when the current impact was rated with the Impact of Event Scale, carriers gave a more negative estimate of the impact of the result than non-carriers (12.7 versus 8.1 on a scale of 45, P=0.008). They also reported less ability to cope with the results than non-carriers (64 versus 27%) (P=0.0006). It is noteworthy, however, that more than a quarter of the non-carriers reported difficulty coping despite the favourable results of the genetic test.
Discussion
We assessed the outcome after presymptomatic testing for Huntington disease in 119 testees. There was a response rate of 40%. A comparable recent study reported the same response rate.11 We showed that carriers feel more hopeless and tend to be more depressed than non-carriers. Current depression, even after a mean delay of 3.7 years after the test result, was frequent, affecting 58% of the carriers and 24% of the non-carriers. These data are robust because we used not only self-reporting scales but also the MINI interview when depression was suspected. In carriers the depression could reflect the first manifestations of the disease. This interpretation has been corroborated by a follow- up study in which the authors conclude that the rate of depression increased as a function of proximity to clinical onset.23 Nevertheless, our data does not fully agree with this finding because non-carriers were also substantially depressed. The rate of depression in non-carriers was higher than the rate of mood disorders in the French population (6.7%, 95% IC: 5.8–7.6).24, 25 Access to specific care evidenced by the use of psychotropic drugs was low, but similar to that reported in the French population.25 The high rate of depression after testing in non-carriers might, therefore, be related to their personal history, and is reflected in the frequency of suicide attempts and hospitalization in psychiatric departments reported by non-carriers and carriers. These results indicate that an unfavourable outcome is related more to a prior history of depression rather than to a ‘good' or ‘bad' test result. This was already reported in Swedish testees with high suicidal ideation and attempts in both groups.26 They noticed that even relatives of both had high frequency of psychiatric disease, pointing at a potential role of familial burden related to HD.
In our study we tested several variables, which could precipitate depression after the result. Interestingly, three factors determinate the fact being depressed after the result: being carrier, having taken the test because of other motivations than for the offspring or a parental project and having a history of depression before the test. There could be a bias in this statement, because we assessed the pre-test history of depression retrospectively. Regarding test motivation this was noted by Decruyenaere et al8 who showed unspecified motivation was a strong predictor of post test distress.
We found that the feeling of hopelessness (‘no future'), which was more frequent in carriers than in non-carriers, did not generate more anxiety in carriers than in non-carriers, and was moderate in both. It has been shown that hopelessness decreased dramatically shortly after the test, remained stable for 3 years,27 but long-term follow up (7–10 years after the result) showed that hopelessness increased again.11
Several studies showed that, both depression and anxiety, improved over time in non-carriers.8, 9, 13
Coping was adapted to the nature of the result. We showed that 52% of non-carriers had efficient coping strategies and 64% of the carriers had not efficient coping. But interestingly, 27% of the non-carriers also had inefficient coping, whereas 18% of the carriers had efficient coping. These paradoxical reactions suggest that psychological follow-up is necessary, even for persons who have received a favourable result of their presymptomatic test. One reason for negative reactions of confirmed non-carriers might be an important change in personal identity of someone who had lived with the idea that he had a serious disease. In any case, non-carriers need time to recover from being ‘at risk'. Five years were reported to be necessary for an improvement in the quality of life of such persons.8, 13 This is comparable to the emotional state of mourning for the loss of being at risk and the doubt about one's genetic status. That unexpected result causes suffering is also reflected by the impact score, which was similar in carriers and non-carriers. Even after a long delay, the person who is no longer at risk may have difficulty living with the favourable result. We could hypothesize: a) guilt about surviving without the ‘bad' gene; b) regret for life decisions made in the past as a function of the risk; c) inability to leave behind the at risk status; d) inability to believe the result and e) reactions of familial environment.
In addition to psychological aid for carriers, those who begin to show symptoms also need special care. Carriers who had neurological signs were as depressed as asymptomatic carriers (75 versus 58% P=0.7), suggesting that a depressed mood is part of the disease at onset and the risk of suicidal behaviour might be greatest at this moment. In a worldwide survey all persons who committed suicide had HD and 52% of those who attempted suicide were symptomatic.2 It is therefore very important to detect the first subtle non-motor changes of the disease, which are indicative of depression and possible adverse reactions.
Unexpectedly, the results of presymptomatic testing had no impact on the social adjustment or carriers or non-carriers, who both had scores which were similar to those obtained with the normal population.
Conclusion
Psychological support and psychiatric care should be given to both carriers and non-carriers after presymptomatic testing for Huntington disease. Particularly and regardless of the result, a history of depression before the test and previous familial burden of psychiatric events will influence the outcome after the test.
Acknowledgments
Thanks to Alexis Brice and Merle Ruberg for fruitful discussions and critical reading of the paper. SL had received a fellowship from the Association Huntington France.
References
- Guidelines for the molecular genetics predictive test in Huntington's disease International Huntington Association (IHA) and the World Federation of Neurology (WFN) Research Group on Huntington's Chorea. Neurology. 1994;44:1533–1536. [PubMed] [Google Scholar]
- Almqvist EW, Bloch M, Brinkman R, Craufurd D, Hayden MR. A worldwide assessment of the frequency of suicide, suicide attempts, or psychiatric hospitalization after predictive testing for Huntington disease. Am J Hum Genet. 1999;64:1293–1304. doi: 10.1086/302374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers-Kiebooms G, Decruyenaere M. Predictive testing for Huntington's disease: a challenge for persons at risk and for professionals. Patient Educ Couns. 1998;35:15–26. doi: 10.1016/s0738-3991(98)00086-x. [DOI] [PubMed] [Google Scholar]
- Goizet C, Lesca G, Durr A. Presymptomatic testing in Huntington's disease and autosomal dominant cerebellar ataxias. Neurology. 2002;59:1330–1336. doi: 10.1212/01.wnl.0000032255.75650.c2. [DOI] [PubMed] [Google Scholar]
- Mandich P, Jacopini G, Di Maria E, et al. Predictive testing for Huntington's disease: ten years' experience in two Italian centres. Ital J Neurol Sci. 1998;19:68–74. doi: 10.1007/BF02427559. [DOI] [PubMed] [Google Scholar]
- Decruyenaere M, Evers-Kiebooms G, Boogaerts A, et al. Predictive testing for Huntington's disease: risk perception, reasons for testing and psychological profile of test applicants. Genet Couns. 1995;6:1–13. [PubMed] [Google Scholar]
- Decruyenaere M, Evers-Kiebooms G, Boogaerts A, et al. Prediction of psychological functioning one year after the predictive test for Huntington's disease and impact of the test result on reproductive decision making. J Med Genet. 1996;33:737–743. doi: 10.1136/jmg.33.9.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decruyenaere M, Evers-Kiebooms G, Cloostermans T, et al. Psychological distress in the 5-year period after predictive testing for Huntington's disease. Eur J Hum Genet. 2003;11:30–38. doi: 10.1038/sj.ejhg.5200913. [DOI] [PubMed] [Google Scholar]
- Duisterhof M, Trijsburg RW, Niermeijer MF, Roos RA, Tibben A. Psychological studies in Huntington's disease: making up the balance. J Med Genet. 2001;38:852–861. doi: 10.1136/jmg.38.12.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibben A, Duivenvoorden HJ, Niermeijer MF, Vegter-van der Vlis M, Roos RA, Verhage F. Psychological effects of presymptomatic DNA testing for Huntington's disease in the Dutch program. Psychosom Med. 1994;56:526–532. doi: 10.1097/00006842-199411000-00008. [DOI] [PubMed] [Google Scholar]
- Timman R, Roos R, Maat-Kievit A, Tibben A. Adverse effects of predictive testing for Huntington disease underestimated: long-term effects 7–10 years after the test. Health Psychol. 2004;23:189–197. doi: 10.1037/0278-6133.23.2.189. [DOI] [PubMed] [Google Scholar]
- Wiggins S, Whyte P, Huggins M, et al. The psychological consequences of predictive testing for Huntington's disease. Canadian Collaborative Study of Predictive Testing. N Engl J Med. 1992;327:1401–1405. doi: 10.1056/NEJM199211123272001. [DOI] [PubMed] [Google Scholar]
- Almqvist EW, Brinkman RR, Wiggins S, Hayden MR. Psychological consequences and predictors of adverse events in the first 5 years after predictive testing for Huntington's disease. Clin Genet. 2003;64:300–309. doi: 10.1034/j.1399-0004.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- Dode C, Durr A, Pecheux C, et al. Huntington's disease in French families: CAG repeat expansion and linkage disequilibrium analysis. C R Acad Sci III. 1993;316:1374–1380. [PubMed] [Google Scholar]
- Amiel-Lebigre F. [Existential events and psychopathology] Encephale. 1983;9:9B–16B. [PubMed] [Google Scholar]
- Lazarus R. Emotion and Adaptation. New York: Oxford University Press; 1991. [Google Scholar]
- Collet L, Cottraux J. [The shortened Beck depression inventory (13 items). Study of the concurrent validity with the Hamilton scale and Widlocher's retardation scale] Encephale. 1986;12:77–79. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10 J Clin Psychiatry 19985922–33.quiz 34–57. [PubMed] [Google Scholar]
- Beck AT, Steer RA, Kovacs M, Garrison B. Hopelessness and eventual suicide: a 10-year prospective study of patients hospitalized with suicidal ideation. Am J Psychiatry. 1985;142:559–563. doi: 10.1176/ajp.142.5.559. [DOI] [PubMed] [Google Scholar]
- Hodgues WF, Spielberger CD. An indicant of trait or state anxiety. J Consult Clin Psychol. 1969;33:430–434. doi: 10.1037/h0027813. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Prusoff BA, Thompson WD, Harding PS, Myers JK. Social adjustment by self-report in a community sample and in psychiatric outpatients. J Nerv Ment Dis. 1978;166:317–326. doi: 10.1097/00005053-197805000-00002. [DOI] [PubMed] [Google Scholar]
- Julien CL, Thompson JC, Wild S, et al. Psychiatric disorders in preclinical Huntington's disease. J Neurol Neurosurg Psychiatry. 2007;78:939–943. doi: 10.1136/jnnp.2006.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyttenaere K, Bruffaerts R, Posada-Villa J, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291:2581–2590. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- Lepine JP, Gasquet I, Kovess V, et al. [Prevalence and comorbidity of psychiatric disorders in the French general population] Encephale. 2005;31:182–194. doi: 10.1016/s0013-7006(05)82385-1. [DOI] [PubMed] [Google Scholar]
- Robins Wahlin TB, Backman L, Lundin A, Haegermark A, Winblad B, Anvret M. High suicidal ideation in persons testing for Huntington's disease. Acta Neurol Scand. 2000;102:150–161. doi: 10.1034/j.1600-0404.2000.102003150.x. [DOI] [PubMed] [Google Scholar]
- Tibben A, Timman R, Bannink EC, Duivenvoorden HJ. Three-year follow-up after presymptomatic testing for Huntington's disease in tested individuals and partners. Health Psychol. 1997;16:20–35. doi: 10.1037//0278-6133.16.1.20. [DOI] [PubMed] [Google Scholar]