Abstract
DNA double-strand repair factors in the non-homologous end joining (NHEJ) pathway resolve DNA double-strand breaks introduced by the recombination-activating gene (RAG) proteins during V(D)J recombination of T and B lymphocyte receptor genes. Defective NHEJ and subsequent failure of V(D)J recombination leads to severe combined immunodeficiency disease (SCID). We originally linked T−B−NK+ SCID in Athabascan-speaking Native Americans in the Southwestern US and Northwest Territories of Canada to chromosome 10. However, despite a common ancestry, the null mutation in the Artemis gene that we found to be causal in the SCID among the Navajo and Apache Indians was not present in the Dine Indians in the Northwest Territories. We now report a novel homozygous missense mutation (R776W) in RAG-1 in three children with T−B−NK+ SCID from two related families of Athabascan-speaking Dine Indians in the Canadian Northwest Territories. As expected, we found no increased sensitivity to ionizing radiation in patient fibroblasts. The impaired activity of this RAG-1 mutant in V(D)J recombination was confirmed by the EGFP-based V(D)J recombination assays. Overexpression of wild type RAG-1 in patient fibroblasts complemented V(D)J recombination, with recovery of both coding and signal joint formation. Our results indicate that the novel R776W missense mutation in RAG-1 is causal in the T−B−NK+ SCID phenotype in Athabascan-speaking Dine Indians from the Canadian Northwest Territories.
Keywords: RAG, SCID, Athabascan-speaking, Artemis
Introduction
Differentiation of T and B lymphocytes requires successful V(D)J recombination by which the germline components, V(Variable), D(Diversity), and J(Joining) gene segments are assembled to encode for V regions of Ig and TCR. It is well established that the RAG-1 and RAG-2 complex initiates V(D)J recombination in lymphoid precursor cells by cleaving at the conserved recombination signal sequences (RSS) that flank the germline V, D, and J coding elements, leading to two blunt signal ends (SE) and two covalently sealed (hairpinned) coding ends (CE). The rejoining of SE or CE is performed by the non-homologous end joining (NHEJ) machinery, which also plays a major role in DNA double-strand break repair in mammalian cells. The well-characterized factors in this pathway consist of Ku heterodimer complex (Ku70/86), DNA-dependent protein kinase catalytic subunit (DNA-PKcs), Artemis, DNA ligase IV, XRCC4, and the more recently identified XRCC4-like factor (XLF).1, 2, 3, 4, 5 The importance of NHEJ processes in mammalian immunogenesis and DNA repair have been described in animal models and some rare types of human severe combined immunodeficiency disease (SCID) caused by germline mutations/deletions in essential NHEJ genes.4, 6, 7, 8, 9, 10, 11, 12
SCID is an immunological disorder primarily arising from inherited mutations, and characterized by severe T cell and B cell immunodeficiency. The T−B−NK+ SCID phenotype make up ∼20% of all reported cases and have a profound absence of both T and B cells but functional NK cells.7 This phenotype is associated with many nonsense and missense mutations, including those in RAG-1 and RAG-2 that initiate V(D)J recombination;9 Artemis, that is involved in hairpin opening during V(D)J recombination;5 Ligase IV that mediates the rejoining of breaks formed during V(D)J recombination.11
Navajo and Apache Native Americans are believed to originate from the Na-Dene subdivision of the Athabascan-speaking linguistic group having migrated into the southwestern US from Alaska and Western Canada between 700 and 1300 AD.13 They have a very high incidence (1:2000 live births) of T−B−NK+ SCID. This Athabascan SCID (SCIDA) displays a particular T−B−NK+ immunophenotype, characterized by defective V(D)J coding joint formation and increased sensitivity to ionizing radiation. Our original linkage study mapped the SCIDA genetic defect to chromosome 10p13 and we subsequently identified a founder mutation in Artemis as the cause of SCIDA in Navajo and Apache Native Americans.8, 14 Surprisingly, this Artemis mutation was not found in the three T−B−NK+ SCID children from two related kindreds of Dine Indians in the Canadian Northwest Territories, a linguistically and genetically related people.
We now report a novel mutation in RAG-1 (R776W) that is homozygous in these three Athabascan-speaking Dine children. This mutation is located in a highly conserved region of RAG-1, and we show that this mutation results in a severe defect in V(D)J recombination.
Materials and methods
BrdU incorporation-based proliferation assays
Fibroblast lines were established from skin biopsies from SCIDA patients 04 and 05, Dine SCID patients D-01 and D-05 and an unrelated immunologically normal individual (AK). Cells were grown in Dulbecco's modified Eagle's medium F12 (with glutamax) (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 1 m sodium pyruvate, 10 m HEPES, and 100 U/ml penicillin/streptomycin in humidified 5% CO2 at 37°C (UCSF Cell Culture Facility). Cells were irradiated using a Pantak® X-ray generator (PANTAK Ltd, East Haven, CT, USA) operating at 320 kV/10 mA with 0.5 mm copper filtration at 0, 1, 3, 5 Gy, washed three times with PBS, and then labeled by the addition of fresh medium containing 10 μg/ml bromodeoxyuridine (BrdU) (Sigma Chemical Co., St Louis, MO, USA) for 24 h. Cells were harvested, fixed, and stained using standard procedures,15 and cell cycle distribution and BrdU incorporation were analyzed with Beckman-Coulter EPICS XL-MCL flow cytometer using XL Data Acquisition software (Beckman Coulter, CA, USA) and WinMDI 2.8 software packages (Joseph Trotter, Scripps, San Diego, CA, USA). The fraction of proliferating cells was calculated by scoring the percent of intact cells staining positive for BrdU and normalizing to the untreated control of the same cell line (≥10 000 events were scored for each point).15
Mutation analysis
Genomic DNA was extracted from cell samples by using Flexigene DNA Kit (Qiagen, Valencia, CA, USA). The coding sequences, cds, of RAG-1 and Artemis were amplified by standard high fidelity PCR protocols as reported earlier.16, 17 Variants were sequenced on ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster city, CA, USA) and sequences were assembled using Gene Tool 2.0 for sequence analysis (BioTools Incorporated, Edmonton, AB, Canada). Variants were compared with the Human Gene Mutation Database (http://archive.uwcm.ac.uk/uwcm/mg/hgmd) to determine whether the mutation had been previously reported. Similarity comparison and possible damage of mutants were evaluated by using the PolyPhen: prediction of functional effect of human nsSNPs (http://genetics.bwh.harvard.edu/pph/).
Generation of constructs
The fragment of the human RAG-1 cDNA (GenBank entry M29474) was cloned into pcDNA6/myc-His Version A (Invitrogen, Carlsbad, CA, USA) to generate the RAG-1-expressing plasmid pWTRAG-1; For construction of pWTRAG-2, the fragment of the human RAG-2 cDNA (GenBank entry M94633) was inserted into pcDNA6/myc-His construct. The expression vectors for the RAG-1 mutants G2276A (pG2276ARAG-1) were also described previously.18 Site-directed mutagenesis on pWTRAG-1 was performed using the Quick Change site-directed Mutagenesis kit (Stratagene, La Jolla, CA, USA) to generate a pC2438TRAG-1 construct. (RAG-1-C2438T-F: 5′-CCATGAGTCTGTGGAAGAACTGTGGGATCGGGTGAAAGGGGTCTC-3′; RAG-1-C2438T-R: 5′-GAGACCCCTTTCACCCGATCCCACAGTTCTTCCACAGACTCATGG-3′). We confirmed the presence of the mutation by using direct sequencing. The EGFP-based inversional V(D)J recombination substrate pE19HK was described containing an EGFP cassette flanked by the recombination signal sequences in opposite direction to the EF-1α promoter and a cassette coding for a hemagglutinin A epitope (HA). pE50HK was used as a positive control for EGFP and HA expression.18
EGFP-based inversional V(D)J recombination assay
The EGFP based V(D)J recombination assay was performed in HEK (human embryonic kidney) 293 cells by cotransfecting pWTRAG-1/2 constructs and the pE19HK substrate by using Lipofectamine™ (Invitrogen, Carlsbad, CA, USA). pC2438TRAG-1 was cotransfected with pWTRAG-2 as well as pE19HK substrate to test the efficiency of V(D)J recombination in comparison with pG2276ARAG-1 (a gift from Dr Klaus Schwarz, University of Ulm, Germany). The transfected cells conferred HA-positive as designed. As V(D)J recombination led to the inversion of the EGFP cassette which was originally inserted in the constructs opposed to its promoter, the corresponding cells turned green once V(D)J recombination occurred. Therefore, the percentage of V(D)J recombination was determined by the ratio of dually positive signals (EGFP and HA) to signals positive solely for HA.18
Extrachromosomal signal-joint and coding-joint assay
The extrachromosomal V(D)J recombination assay and functional complementation were performed in SV40-transformed fibroblasts from patients, HEK 293 by cotransfecting pWTRAG-1/2 constructs and the extrachromosomal substrate pGG49 (ampr for signal joint) or pGG51 (ampr for coding joint) (a gift from Dr Michael Lieber, University of Southern California, USA), using a previously described method.14 pGG49 and pGG51 carry a cam gene that was interrupted from its promoter by a transcriptional terminator flanked by RSSs. Upon transfection, RAG proteins induced V(D)J recombination in the extrachromosomal plasmid substrates, resulting in the excision of the transcriptional terminator and the activation of chloramphenicol resistance. The percentage of successful recombination was represented by the ratio of colonies grown on ampicillin and chloramphenicol (from recombined substrate only) vs ampicillin plates.14
Western blot
Expression of pWTRAG-1, pC2438TRAG-1 was identified by western blotting using Rabbit anti-human RAG-1 (H300) antibody raised against the C-terminal of the human RAG-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Whole cell lysates were prepared from the transfected cell lines, resolved in a pre-cast 10% SDS-PAGE gel system (Invitrogen, Carlsbad, CA, USA), transferred to nitrocellulose membranes, and hybridized with a Rabbit anti-human RAG-1 (H300) antibody (1:1000) and Goat anti-Rabbit IgG, horseradish peroxidase conjugate (Santa Cruz Biotechnology, Santa Cruz, CA, USA), then detection was performed by using ECL western blotting system (Amersham, Piscataway, NJ, USA). Mouse Anti-c-Myc Ab (9E10) (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as an internal control.
Statistics
EGFP-based V(D)J recombination data were analyzed by the Wilcoxon rank sum test and presented as box plots.
Results
Case presentations
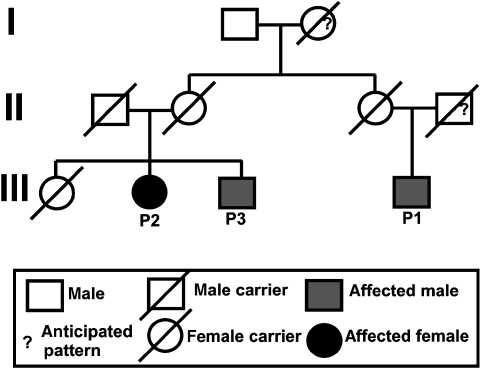
Three children from two related families from the Dine Indians, a linguistic subgroup of Athabascan-speaking Native Americans, were diagnosed with SCID. Of these, two surviving children were further characterized as having T−B−NK+ SCID (Table 1). The families are related through the maternal line, but the degree of consanguinity is not clear. The family history is shown in the pedigree in Figure 1. One ‘unaffected' sibling and three of four parents are still alive.
Table 1. Immunological characteristics of three dine SCID patients.
| Test (normal values) | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| ALC (3.7–9.6 × 109/l) | 0.66 | 0.06 | 0.46 |
| CD3 (53–84%) | <1% | 2% | 2% |
| CD4 (27–56%) | N/D | <1% | 1% |
| CD8 (11–37%) | N/D | <1% | 3% |
| CD19 (6–24%) | N/D | 3.7% | 2% |
| CD16 (8–23%) | N/D | N/D | 57% |
| IgG (176–601 mg/dl) | 114 | 1170 | 196 |
| IgA (3–73 mg/dl) | <17 | 11 | 11 |
| IgM (17–105 mg/dl) | <15 | <6 | 15 |
| PHA (65 189±651 c.p.m.) | 0 | 176±1.4 | 0 |
| Con A (17 745±177 c.p.m.) | N/D | 617±5.0 | N/D |
| PWM (15 578±109 c.p.m.) | N/D | 1910±9.5 | N/D |
N/D, not done; ALC, absolute lymphocyte count at diagnosis.
Figure 1.
A pedigree of a Dine family showing three generations with the SCID children indicated P1, P2, and P3. The ?-symbol indicates genotypes not experimentally determined with DNA sequencing but genetically indicated.
Patient 1 presented with repeated infections with chronic diarrhea and oral Candidiasis. He received an unirradiated cytomegalovirus (CMV)-positive blood transfusion before transfer to UCSF on 10 January 1984 at 3 months of age. He had respiratory distress and jaundice with lymphopenia, thrombocytopenia and low to absent immunoglobulin levels. He was diagnosed with hyperacute GvHD and CMV pneumonia and died 5 days following admission.
Patient 2 was admitted to a local hospital for right upper lobe pneumonia, labial, buccal, and tongue ulcerations and bloody diarrhea. A stool culture was positive for Enterovirus. She was severely lymphopenic and found to have a monoclonal gammopathy and low immunoglobulin levels (Table 1). T−B− NK+SCID was diagnosed and she was transferred to UCSF for a bone marrow transplant. At 5 months of age she received a bone marrow transplant from her HLA-matched father following conditioning with horse anti-thymocyte globulin (ATG). She did well following her transplant and reconstituted T but not B cells, for which she continues to receive regular IVIG infusions.
Patient 3 is the younger brother of patient 2. Because of the positive family history for SCID and an occurrence of thrush early in life, he was evaluated and found to have T−B−NK+ SCID (Table 1). He was admitted to UCSF at 2½ months of age for a bone marrow transplant from a healthy HLA-matched sister following ATG. Post transplant he did quite well with T but not B cell immune reconstitution and is alive and well on IVIG.
Dine SCID patient fibroblasts are not radiation sensitive
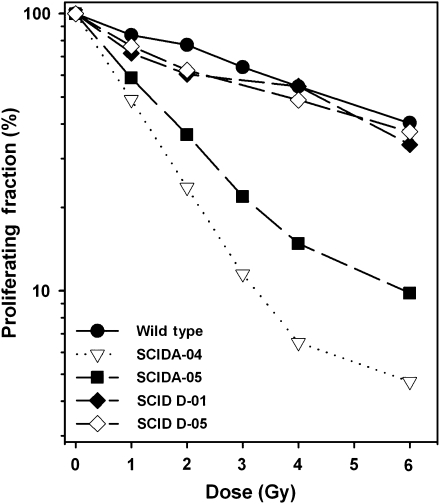
Because the three Dine SCID patients are from Athabascan-speaking populations and showed phenotypic similarities with SCIDA including mouth and genital ulcers and the T−B−NK+ phenotype, we used the BrdU incorporation-based proliferation assay to evaluate the relative radiation sensitivity of their skin fibroblasts.19 Unlike Artemis-deficient fibroblasts from children with SCIDA, we found that Dine SCID patient fibroblasts D-01 and D-05, derived from patients 2 and 3 respectively, exhibited no more sensitivity to ionizing radiation than the wild-type control (Figure 2).
Figure 2.
Radiation sensitivity of SV40 immortalized skin fibroblasts from SCID patients. SCIDA 04, 05 cell lines were known Artemis-deficient lines and SCID D-01 and D-05 were fibroblasts originating from Dine SCID patients 2 and 3 respectively. The proliferating fraction of SV40-immortalized skin fibroblasts was plotted as a function of X-ray dose (Gy) and these data are from three biological replicates.
Identification of a R776W mutation in RAG-1 of Dine SCID patients
Given that these patients have a T−B−NK+ phenotype without radiation sensitivity, we suspected RAG-gene defects as causal in Dine SCID. Sequence analysis of RAG-1 and RAG-2 in these three Dine SCID patients revealed a new homozygous C2438T missense mutation (Figure 3a), which results in an arginine to tryptophan substitution at the position 776 (R776W). Both sets of parents were found to be heterozygous for the C2438T mutation, consistent with the homozygosity of this mutation in the patients. In addition, an unaffected sibling was also heterozygous for the C2438T mutation. The grandfather had no mutation at this position indicating that the grandmother must have been a carrier (Figure 1).
Figure 3.
(a) Sequence analysis of RAG-1 gene in two related families. A novel homozygous single base pair missense mutation was identified (C2438T) in RAG-1 among all three Dine SCID patients, this mutation encodes an arginine to tryptophan substitution at position 776 (R776W). Parents and non-affected sibling were found to be heterozygous for the C2438T mutation (see Figure 1). (b) Protein sequence alignment of RAG-1 from 10 selected species (of >100 species inspected) revealing that the arginine at position 776 in the RAG-1 protein is highly conserved.
Sequence alignment and comparison of the RAG-1 genes from over 100 species showed that R776 itself is highly conserved and is located in a C-terminal motif, which is a highly conserved region of RAG-1 (limited alignments shown, Figure 3b). Previous studies have shown this region to be important for RAG protein-DNA binding and dimerization.9 Furthermore, probability analysis of the R776W mutation resulting in functional effects was carried out with the PolyPhen analysis tool20, 21 giving a PSIC score difference of 3.143, suggesting a mutation at this residue is probably damaging.
RAG-1 R776W mutant shows no V(D)J recombination activity
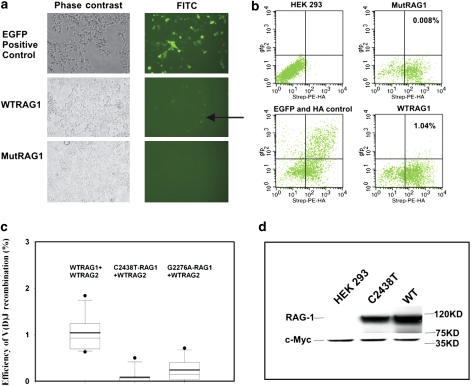
To assess the functional consequence of this newly identified R776W mutation, V(D)J recombination assays were carried out. Wild type (WT) and R776W-RAG-1 expression constructs were cotransfected into HEK 293 cell lines with a RAG-2 expression vector and the V(D)J recombination EGFP-reporter plasmid PE19-HK.18 Inspection of cultures with fluorescence microscopy showed EGFP expression in cells transfected with the positive control vector (p50HK) and the WTRAG-1 indicating successful V(D)J recombination (Figure 4a). In contrast, cotransfection of cells with the R776W-RAG-1 expression vector showed no EGFP-positive cells when scored under the fluorescence microscope. To quantify the efficiencies of V(D)J recombination with either WT or R776W RAG-1 transfections, we carried out FACS analysis on the transfected cultures (Figure 4b). The HEK 293 cells alone had no detectable background whereas cells transfected with the constitutively expressing EGFP vector p50HK, showed >20% positive cells (Figure 4b, left panels). The experimental sets of cells transfected with WT or R776W were stained for HA as a measure of successful transfection, and scored for EGFP as the reporter of successful V(D)J recombination (Figure 4b, right panels). The WTRAG-1 transfection resulted in approximately 1% of the cells staining positive for both HA and EGFP whereas the R776W RAG-1 resulted in 0.008% of cells staining for both HA and EGFP. The difference in V(D)J recombination efficiency between WT and mutant R776W RAG-1 was statistically significant (P<0.05) and comparable to the G2276A RAG-1 mutant reported earlier to be causal in SCID (Figure 4c).18 We evaluated protein expression and found that the C2438T mutant RAG-1 protein is expressed at significant levels as detected by western blot but at consistently lower levels than WTRAG-1 (Figure 4d).
Figure 4.
(a) Visualization of V(D)J recombination positive cells expressing EGFP with fluorescence microscopy. EGFP-positive HEK 293 cells were detected 48 h after transient transfection with pWTRAG-1/2 expression constructs and reported plasmids (see arrow). When WTRAG-1 was replaced with the C2438T mutant RAG-1 construct, no EGFP-positive cells were observed. pE50HK transfected cells served as the EGFP and HA positive control. (b) V(D)J recombination efficiency was analyzed by scoring EGFP/HA-positive cells by FACS. Cotransfection of HEK 293 cells with pC2438T-RAG-1 vector and reporter system plasmids gives minimal V(D)J recombination (0.008%) relative to WTRAG-1 transfections. Experiments were repeated in triplicate. (c) Statistical analysis showed that there was a significant difference between the WTRAG-1 and the C2438T mutant RAG-1 with respect to V(D)J recombination efficiency (P<0.05). (d) C2438T mutant RAG-1 protein is expressed at significant levels as detected by western blot but at consistently lower levels than WTRAG-1.
R776W RAG-1 mutant failed to induce both signal and coding joint formation
To further identify possible functional differences in coding and signal joint formation between the mutant and WTRAG-1, we transfected HEK 293 cells that were transiently expressing RAG-1 (WT or mutant) and RAG-2 with either pGG49 (signal joint) or pGG51 (coding joint) reporter plasmids. Reporter plasmids were recovered after 48 h and used to transform E. coli. Dual-resistant bacterial colonies indicating successful V(D)J recombination were recovered after transfection with WTRAG-1 for coding joint (average >0.3%) and signal joint (average >0.1%). In contrast, transformation of R776W RAG-1 resulted in far fewer dual-resistant colonies with both coding joint and signal joint formation significantly impaired with average rates of <0.0024 in both cases (Table 2).
Table 2. Test of V(D)J recombination driven signal joint and coding joint formation in HEK293 cells.
| Signal (pGG49) | Coding (pGG51) | |||||
|---|---|---|---|---|---|---|
| Plasmid | AmpR Colonies | AmpR/CamR Colonies | Ratea (%) | AmpR Colonies | AmpR/CamR Colonies | Rateb (%) |
| pWTRAG-1 Exp 1 | 124 000 | 288 | 0.23 | 6000 | 48 | 0.8 |
| Exp 2 | 128 000 | 182 | 0.14 | 13 600 | 15 | 0.11 |
| Exp 3 | 78 000 | 29 | 0.04 | 16 000 | 27 | 0.17 |
| PMUTRAG-1 Exp 1 | 110 000 | 0 | 0.00 | 46 000 | 2 | 0.004 |
| Exp 2 | 120 000 | 2 | 0.0016 | 110 000 | 2 | 0.0018 |
| Exp 3 | 58 000 | 0 | 0.00 | 150 000 | 2 | 0.0013 |
AmpR, Ampicillin resistance; CamR, Chromphenicol resistance.
Rate (signal joints)=(AmpR/CamR colonies)/(AmpR colonies) × 100%.
Rate (coding joints)=(AmpR/CamR colonies)/(AmpR colonies) × 100%.
Wild type RAG-1 cDNA complements the Dine SCID V(D)J recombination defect
To confirm that the R776W RAG-1 mutation was responsible for the V(D)J recombination defect observed in Dine SCID, we cloned the WT and R776W RAG-1 cDNA into a mammalian expression vector and assessed its functional complementation activity in the extra chromosomal V(D)J recombination assay in two Dine SCID patient fibroblasts. As expected, coding and signal joint formation was significantly complemented following transfection with WTRAG-1 constructs (Table 3), whereas no double-resistant colonies were detected after transfection with R776W RAG-1 cDNA (not shown). Sequence analysis of plasmids recovered from ampicillin and chloramphenicol-resistant colonies reveal that the junctions were true V(D)J coding and signal joints with limited trimming of joining ends (data not shown).
Table 3. Complementation of V(D)J recombination driven signal and coding joint formation in SCID fibroblasts transfected with WT RAG-1 cDNA.
| Signal (pGG49) | Coding (pGG51) | |||||
|---|---|---|---|---|---|---|
| Cell lines | AmpR colonies | AmpR/CamR colonies | Ratea (%) | AmpR colonies | AmpR/CamR colonies | Rateb (%) |
| 17001 Exp 1 | 11 000 | 20 | 0.18 | 4000 | 12 | 0.30 |
| Exp 2 | 8000 | 12 | 0.15 | 5340 | 6 | 0.11 |
| Exp 3 | 7600 | 16 | 0.20 | 7600 | 8 | 0.105 |
| 17005 Exp 1 | 10 400 | 32 | 0.30 | 5000 | 12 | 0.24 |
| Exp 2 | 8700 | 15 | 0.17 | 4030 | 9 | 0.22 |
| Exp 3 | 9400 | 17 | 0.18 | 3000 | 10 | 0.33 |
AmpR, Ampicillin resistance; CamR, Chromphenicol resistance.
Rate (signal joints)=(AmpR/CamR colonies)/(AmpR colonies) × 100%.
Rate (coding joints)=AmpR/CamR colonies)/(AmpR colonies) × 100%.
Discussion
The R776W RAG-1 mutation resulting in the T−B−NK+ SCID phenotype in Dine SCID was not reported earlier. Compared to the previously reported G2276A RAG-1 mutant,18 both mutations that render V(D)J recombination non-functional are adjacent within the RAG-1 sequence in a highly conserved region of the protein. The mutation at the highly conserved amino-acid position 776 results in abnormal RAG-1 activity, but the exact functional mechanism of the R776W mutation remains unclear. It is conceivable that an amino-acid change at such a conserved region may cause disturbances in RAG-1 protein-DNA binding or its dimerization, or alter the nuclease activity directly. Interestingly, western blot data consistently showed lower levels of the R776W RAG-1 when compared with WTRAG-1, despite identical expression vectors and transfection procedures. Although this does not accurately mimic the physiological situation, it does suggest that the R776W RAG-1 mutant protein is either produced at lower levels or is less stable than the WT (Figure 4d).
We previously demonstrated the absence of V(D)J recombination-derived coding joint with normal signal joint formation in patients with defective Artemis (SCIDA).8, 13 In contrast, this mutation in RAG-1 cannot facilitate signal or coding joint formation, indicating that the R776W RAG-1 either fails to incise DNA or completely blocks subsequent recombination steps in vivo. It appears that this single amino-acid replacement in RAG-1 (R776W) results in a nearly complete failure of V(D)J recombination and the subsequent T and B cell deficiencies and disease. Our molecular results are consistent with the clinical features of these three Dine SCID patients and we can ascribe the V(D)J recombination defect in these patients to the R776W mutation in the RAG-1 gene.
To conclude, this novel homozygous RAG-1 mutation at position 776 which occurred in three Athabascan-speaking Dine children with T−B−NK+ SCID causes the loss of RAG-1 function in V(D)J recombination. The Athabascan SCID (SCIDA) immunophenotype is characterized by defective V(D)J coding joint formation and increased sensitivity to ionizing radiation in fibroblasts. In contrast, our functional assays demonstrate that this homozygous RAG-1 mutation in Dine SCID failed to induce either coding or signal joint formation, also resulting in a disturbance in both T and B cell development. Furthermore, this V(D)J recombination defect in Dine SCID can be complemented by overexpression of wild type RAG-1 cDNA. Unlike SCIDA, which has an impaired NHEJ pathway because of Artemis deficiency, the absence of increased radiation sensitivity in the Dine Indian patients' fibroblasts indicates an intact NHEJ pathway. In addition to the founder mutation of Artemis identified in Navajo and Apache Indians with SCID,8 we have also described the novel occurrence of X-linked SCID in a Navajo kindred with maternal mosaicism for a deleterious mutation of the interleukin common γ chain receptor (IL2RG).22 The novel R776W homozygous RAG-1 mutation is distinct from those described in previous studies and is the third gene causing SCID among Athabascan-speaking Native Americans. The identification of this third immunological mutation in this isolated population underscores the need for newborn screening in this very high-risk population.
Acknowledgments
We acknowledge Randa Ibeid for her excellent technical support. We also thank Dr Klaus Schwarz (University of Ulm, Germany) and Dr Michael Lieber (University of Southern California, USA) for providing us plasmids and valuable expertise. This work was supported in part by an NIH R01 (5 R01 HL058842-07) and an MOD grant (6-FY05-84) and work at LBNL was supported by the US Department of Energy Office of Science, under contract no. DE-AC02-05CH11231 (SMY) and US National Institutes of Health grant CA104660 (SMY). We declare no conflict of interests.
References
- Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109 Suppl:S45–S55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- Fugmann SD. RAG1 and RAG2 in V(D)J recombination and transposition. Immunol Res. 2001;23:23–39. doi: 10.1385/IR:23:1:23. [DOI] [PubMed] [Google Scholar]
- Gellert M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu Rev Biochem. 2002;71:101–132. doi: 10.1146/annurev.biochem.71.090501.150203. [DOI] [PubMed] [Google Scholar]
- Buck D, Malivert L, de Chasseval R, et al. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell. 2006;124:287–299. doi: 10.1016/j.cell.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Ma Y, Pannicke U, Schwarz K, Lieber MR. Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell. 2002;108:781–794. doi: 10.1016/s0092-8674(02)00671-2. [DOI] [PubMed] [Google Scholar]
- Gu Y, Seidl KJ, Rathbun GA, et al. Growth retardation and leaky SCID phenotype of Ku70-deficient mice. Immunity. 1997;7:653–665. doi: 10.1016/s1074-7613(00)80386-6. [DOI] [PubMed] [Google Scholar]
- de Villartay JP, Poinsignon C, de Chasseval R, Buck D, Le Guyader G, Villey I. Human and animal models of V(D)J recombination deficiency. Curr Opin Immunol. 2003;15:592–598. doi: 10.1016/s0952-7915(03)00101-8. [DOI] [PubMed] [Google Scholar]
- Li L, Moshous D, Zhou Y, et al. A founder mutation in Artemis, an SNM1-like protein, causes SCID in Athabascan-speaking Native Americans. J Immunol. 2002;168:6323–6329. doi: 10.4049/jimmunol.168.12.6323. [DOI] [PubMed] [Google Scholar]
- Villa A, Sobacchi C, Notarangelo LD, et al. V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood. 2001;97:81–88. doi: 10.1182/blood.v97.1.81. [DOI] [PubMed] [Google Scholar]
- Gao Y, Chaudhuri J, Zhu C, Davidson L, Weaver DT, Alt FW. A targeted DNA-PKcs-null mutation reveals DNA-PK-independent functions for KU in V(D)J recombination. Immunity. 1998;9:367–376. doi: 10.1016/s1074-7613(00)80619-6. [DOI] [PubMed] [Google Scholar]
- Grawunder U, Zimmer D, Kulesza P, Lieber MR. Requirement for an interaction of XRCC4 with DNA ligase IV for wild-type V(D)J recombination and DNA double-strand break repair in vivo. J Biol Chem. 1998;273:24708–24714. doi: 10.1074/jbc.273.38.24708. [DOI] [PubMed] [Google Scholar]
- Zhu C, Bogue MA, Lim DS, Hasty P, Roth DB. Ku86-deficient mice exhibit severe combined immunodeficiency and defective processing of V(D)J recombination intermediates. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- Li L, Drayna D, Hu D, et al. The gene for severe combined immunodeficiency disease in Athabascan-speaking Native Americans is located on chromosome 10p. Am J Hum Genet. 1998;62:136–144. doi: 10.1086/301688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Salido E, Zhou Y, et al. Targeted disruption of the Artemis murine counterpart results in SCID and defective V(D)J recombination that is partially corrected with bone marrow transplantation. J Immunol. 2005;174:2420–2428. doi: 10.4049/jimmunol.174.4.2420. [DOI] [PubMed] [Google Scholar]
- Wang J, Pluth JM, Cooper PK, Cowan MJ, Chen DJ, Yannone SM. Artemis deficiency confers a DNA double-strand break repair defect and Artemis phosphorylation status is altered by DNA damage and cell cycle progression. DNA Repair (Amst) 2005;4:556–570. doi: 10.1016/j.dnarep.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Moshous D, Callebaut I, de Chasseval R, et al. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell. 2001;105:177–186. doi: 10.1016/s0092-8674(01)00309-9. [DOI] [PubMed] [Google Scholar]
- Schwarz K, Gauss GH, Ludwig L, et al. RAG mutations in human B cell-negative SCID. Science. 1996;274:97–99. doi: 10.1126/science.274.5284.97. [DOI] [PubMed] [Google Scholar]
- Zheng X, Schwarz K. Making V(D)J rearrangement visible: quantification of recombination efficiency in real time at the single cell level. J Immunol Methods. 2006;315:133–143. doi: 10.1016/j.jim.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Povirk LF, Zhou T, Zhou R, Cowan MJ, Yannone SM. Processing of 3′-phosphoglycolate-terminated DNA double strand breaks by Artemis nuclease. J Biol Chem. 2007;282:3547–3558. doi: 10.1074/jbc.M607745200. [DOI] [PubMed] [Google Scholar]
- Sunyaev S, Ramensky V, Koch I, Lathe W, III, Kondrashov AS, Bork P. Prediction of deleterious human alleles. Hum Mol Genet. 2001;10:591–597. doi: 10.1093/hmg/10.6.591. [DOI] [PubMed] [Google Scholar]
- Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Marcaigh AS, Puck JM, Pepper AE, De Santes K, Cowan MJ. Maternal mosaicism for a novel interleukin-2 receptor gamma-chain mutation causing X-linked severe combined immunodeficiency in a Navajo kindred. J Clin Immunol. 1997;17:29–33. doi: 10.1023/a:1027332327827. [DOI] [PubMed] [Google Scholar]