Abstract
The Kallmann syndrome (KS) combines hypogonadotropic hypogonadism (HH) with anosmia. This is a clinically and genetically heterogeneous disease. KAL1, encoding the extracellular glycoprotein anosmin-1, is responsible for the X chromosome-linked recessive form of the disease. Mutations in FGFR1 or FGF8, encoding fibroblast growth factor receptor-1 and fibroblast growth factor-8, respectively, underlie an autosomal dominant form with incomplete penetrance. Finally, mutations in PROKR2 and PROK2, encoding prokineticin receptor-2 and prokineticin-2, have been found in heterozygous, homozygous, and compound heterozygous states. These two genes are likely to be involved both in monogenic recessive and digenic/oligogenic KS transmission modes. Notably, mutations in any of the above-mentioned KS genes have been found in less than 30% of the KS patients, which indicates that other genes involved in the disease remain to be discovered.
Keywords: Kallmann syndrome, KAL1, FGFR1, FGF8, PROKR2, PROK2
In brief
KS is a genetically heterogeneous developmental disease that most often manifests as absent spontaneous puberty combined with a defective sense of smell (hyposmia or anosmia).
Some non-reproductive non-olfactory anomalies can also be present, depending on the genetic form of the disease.
Disease prevalence has been roughly estimated at 1:8000 males and 1:40000 females, but might be underestimated especially in females.
Main differential diagnoses are normosmic idiopathic hypogonadotropic hypogonadism and CHARGE syndrome.
Different modes of KS transmission include X chromosome-linked recessive, autosomal recessive, autosomal dominant with incomplete penetrance, and most probably digenic/oligogenic inheritance.
Mutations in any of the five known disease genes (KAL1, FGFR1, FGF8, PROKR2, PROK2) have been identified in a relatively small proportion (less than 30%) of the patients.
As many as 30% of the mutations found in FGFR1 might be de novo mutations, certainly a possibility to be considered before assessing recurrence risk of this genetic form in a family.
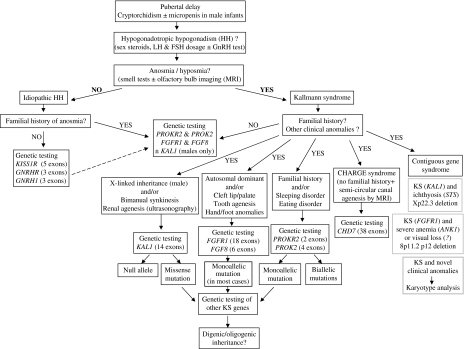
Genetic testing strategy (Figure 1) is based on patient's gender, familial history (if any) and putative mode of disease inheritance, and the presence of additional clinical anomalies that may direct the geneticist towards a particular disease gene or occasionally a contiguous gene syndrome.
Treatment of KS is that of the hypogonadism. There is currently no treatment for olfactory deficit. In both sexes, hormone replacement therapies are used to stimulate the development of secondary sexual characteristics at the time of puberty, and later to induce fertility.
Introduction
Maestre de San Juan was probably the first to report, in 1856, the association of the absence of olfactory structures in the brain and the presence of small testes in an individual.1 The syndrome was identified as a clinical entity in 1944 by an American medical geneticist, Kallmann, who carried out a study on the occurrence of hypogonadism accompanied by anosmia in three affected families.2 He showed the cosegregation of the anosmia and the hypogonadism in all the affected individuals, and therefore established that this syndrome can be hereditary. In the 1950s, the Swiss anatomist de Morsier further documented the disease by describing the underdevelopment or absence of the olfactory bulbs and tracts in several male patients with hypogonadism.3 Some years later, the hypogonadism was ascribed to gonadotropin-releasing hormone (GnRH) deficiency.4
The prevalence of KS is still unknown. It has been roughly estimated at one out of 8000 in boys. In girls, the prevalence is thought to be five times lower, but is probably underestimated because some affected females only have mild hypogonadism (see below). Moreover, primary amenorrhea in females often remains unexplored.
Clinical overview
The Kallmann syndrome typically combines severe HH with a complete absence of the sense of smell (anosmia). The degree of the hypogonadism and that of the smell deficiency can, however, vary significantly, not only between unrelated patients, but also within affected families5, 6 (and see pedigrees in references7, 8, 9, 10, 11, 12, 13, 14), even between monozygotic twins.15, 16 In some families, both typical KS phenotypes and dissociated phenotypes with either hypogonadism or anosmia have been described.7, 10, 13, 14, 17 In addition, apparent reversal of the hypogonadism after discontinuation of hormonal treatment has been reported in a few KS patients.9, 18, 19 Finally, a variety of non-reproductive non-olfactory additional anomalies are present in only a fraction of KS patients. These disorders include involuntary upper limb mirror movements (bimanual synkinesis),17, 20, 21, 22 abnormal eye movements,21, 23 congenital ptosis,24, 25 abnormal visual spatial attention,26 hearing impairment,5, 6, 8, 27, 28, 29 agenesis of the corpus callosum,7, 13 unilateral (occasionally bilateral) renal agenesis,30, 31, 32 cleft lip or palate,5, 6, 7, 33 agenesis of one or several teeth (hypodontia),7, 24, 33, 34 obesity6, 10 and other less documented anomalies (see reference35 for review).
Differential diagnosis: normosmic idiopathic HH and CHARGE syndrome
Difficulties are encountered at both ends of KS phenotypic spectrum that is either in the absence of a conspicuous smell deficiency or when non-reproductive non-olfactory additional anomalies are present on top of a typical KS (see Figure 1).
Figure 1.
Genetic testing strategy for Kallmann syndrome. The strategy is based on patient's gender, familial history (if any) and putative mode of disease inheritance, and the presence of additional clinical anomalies that may direct the geneticist towards a particular disease gene or, occasionally, a contiguous gene syndrome at Xp22.336 or 8p11.2 p12.7 The search for KAL1 mutations is restricted to affected males, either isolated cases or patients with a familial history compatible with X-linked recessive mode of inheritance. Mutation screening of the known KS genes (KAL1, FGFR1, FGF8, PROKR2, PROK2) leads to the identification of a mutation in less than one-third of the patients. Notably, as many as 30% of the mutations found in FGFR1 might be de novo mutations, certainly a possibility to be considered before assessing recurrence risk of this genetic form in a family. The main differential diagnoses of KS are normosmic idiopathic hypogonadotropic hypogonadism and CHARGE syndrome.
Given the variable degree of hyposmia in KS, the distinction between KS and normosmic idiopathic HH (nIHH) is currently unclear, especially as HH patients do not always undergo detailed olfactory testing. There is genetic evidence, however, to suggest that nIHH and KS represent distinct nosological entities. Indeed, the genes encoding GnRH and kisspeptin receptors that are involved in nIHH37, 38, 39 do not seem to be required for the embryonic migration of neuroendocrine GnRH cells, the process likely to be defective in KS patients (see below). Large scale genetic testing of genuine nIHH cases for the presence of mutations in KS genes should help to clarify this issue. The recent report of a family in which deleterious GNRHR and FGFR1 missense mutations cosegregated in the nIHH individuals indicates, however, that the situation could be more complicated than anticipated.40
CHARGE syndrome has an estimated birth incidence of 1 in 8500–12 000. The defining features that make the acronym are coloboma, heart anomalies, choanal atresia, retardation of growth and/or development, genital and ear anomalies. However, no single feature is universally present or sufficient for the diagnosis of CHARGE syndrome. Other frequently occurring features include characteristic face and hand dysmorphia, hypotonia, arhinencephaly, semicircular canal agenesis or hypoplasia, hearing impairment, urinary tract anomalies, orofacial clefting, dysphagia, and tracheo-oesophagial anomalies. New diagnostic criteria have been proposed in the past few years (see reference41). Moreover, it has been reported that most if not all CHARGE patients have both olfactory bulb aplasia or hypoplasia and HH,42, 43 that is, the two KS defining features. Consequently, previously reported KS cases associated with congenital heart disease44 or choanal atresia45 could in fact represent unrecognised mild CHARGE cases.46 CHARGE syndrome shares additional traits with the KAL2 genetic form of KS (see below), including cleft lip or palate, present in 20–35% of KAL27, 11, 12, 13 and CHARGE47 patients, external ear malformation, noted in virtually all CHARGE patients47 and a few KAL2 patients,48 agenesis of the corpus callosum, reported in several CHARGE47 and KAL2 patients,7, 13 and coloboma that is highly prevalent in CHARGE patients47 and has been reported in at least one KAL2 patient too.7 Most individuals with CHARGE syndrome are heterozygous for loss-of-function mutations in CHD7 that encodes a chromodomain (chromatin organisation modifier domain) helicase DNA-binding protein.49, 50 Because of the similarity between CHARGE and KAL2 phenotypes, it is tempting to speculate that there are functional interactions between CHD7 and the FGFR1-signalling pathway.
Diagnostic approaches
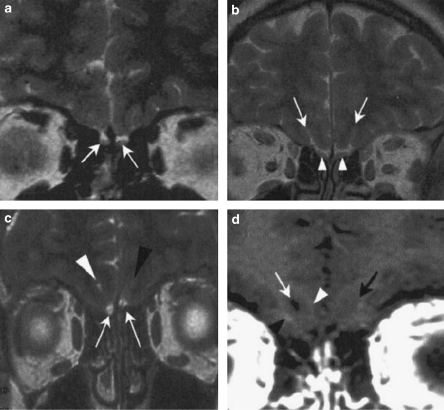
Most cases are diagnosed at the time of puberty because of the lack of sexual development, identified by small testes and absent virilisation in males or the lack of breast development and primary amenorrhea in females. KS is diagnosed when low serum gonadotropins and gonadal steroids are coupled with a compromised sense of smell. The latter should be ascertained by means of detailed questioning and olfactory screening tests,51, 52, 53, 54 because it is rarely mentioned spontaneously. Magnetic resonance imaging (MRI) of the forebrain can be carried out to show the hypoplasia or aplasia of the olfactory bulbs and tracts55 (Figure 2). MRI is also useful to exclude hypothalamic or pituitary lesions as the cause of HH.56 The GnRH deficiency can be indirectly assessed by means of endocrinological tests (see reference57).
Figure 2.
Cranial magnetic resonance imaging (MRI) of the olfactory bulb region in a control man (a and b) and a man affected by Kallmann syndrome (c and d). (a and b) MRI T2-weighted sequence in coronal plane shows normal olfactory bulbs in the control individual (a, white arrows), and posterior to the olfactory bulbs, good differentiation of the rhinal sulci (b, white arrows) and olfactory tracts (b, white arrowheads). (c) MRI T2-weighted sequence in coronal plane shows very small olfactory bulbs in the KS patient (white arrows). On the right side, the rhinal sulcus (white arrowhead) is visible, with good differentiation between the right gyrus and orbital gyrus. On the left side, there is no rhinal sulcus (black arrowhead), and no differentiation between the right and orbital gyri. (d) MRI T1-weighted sequence in coronal plane, posterior to olfactory bulbs, confirms the presence of the rhinal sulcus on the right side (white arrow), with a relatively good differentiation between the right (white arrowhead) and orbital (black arrowhead) gyri, and the absence of the rhinal sulcus on the left side (black arrow).
Notably, KS may also be suspected as early as in infancy in boys, in the presence of cryptorchidism or a micropenis, combined with subnormal LH and FSH concentrations. Indeed, the postnatal surge in FSH, LH, and testosterone in the male infant as a consequence of the continued function of the fetal GnRH pulse generator provides a 6-month window of opportunity to establish the diagnosis of HH,58 and alert the clinician to the possibility of its association with olfactory impairment. In this respect, the usefulness of forebrain MRI in diagnosing the disease in children too young to undergo meaningful testing of olfaction or of the hypothalamo-pituitary-gonadal axis should be emphasised,59, 60 even though normal olfactory bulb images have been reported in a few KS patients.22, 29
Finally, the presence of non-reproductive non-olfactory additional disorders, including mirror movements, palate anomalies, renal agenesis (ultrasonography), hearing impairment (audiometric testing), and tooth agenesis, should be carefully searched in the patients and, whenever possible, their first-degree relatives, because such anomalies can direct the geneticist towards particular genetic forms of the disease (see below and Figure 1). In KS-affected families, the cleft palate or renal agenesis diagnosed by means of fetal ultrasonography may occasionally reveal the disease before birth.
The complex genetics of KS
Although most KS patients present as sporadic cases, many cases are clearly familial, with three modes of inheritance being reported: X chromosome-linked recessive (OMIM no. 308700), autosomal dominant (OMIM no. 147950), and autosomal recessive (OMIM no. 244200) (http://www.ncbi.nlm.nih.gov/omim/). In the autosomal dominant form, incomplete penetrance has been emphasized.5, 61
Five causal genes have been identified to date, namely, by chronological order of discovery, KAL1,62, 63, 64 FGFR1,7 PROKR2 and PROK2,10 and FGF8.65 Various loss-of-function mutations in KAL1, encoding the extracellular matrix glycoprotein anosmin-1, and in FGFR1 or FGF8, encoding fibroblast growth factor receptor-1 and fibroblast growth factor-8, underlie the X chromosome-linked form (KAL1) and an autosomal dominant form (KAL2) of KS, respectively (see Supplementary Tables S1, S2 and S3 for a list of the mutations). The KAL1 and KAL2 genetic forms account for roughly 8% and 10% of all KS cases, respectively. Mutations in KAL1 are mainly nonsense mutations, frameshift mutations, or large gene deletions, whereas the majority of mutations in FGFR1 (ie, approximately 70%) or FGF8 (all six mutations reported so far) are missense mutations. Notably, as many as 30% of the FGFR1 mutations found in the patients could be de novo mutations13, 48, 66 (C Dodé, unpublished results). Putative loss-of-function mutations in PROKR2 or PROK2, encoding prokineticin receptor-2 and prokineticin-2, respectively, have been detected in approximately 9% of the KS patients (see Supplementary Table S4 for a list of the mutations). Most of these mutations are missense mutations, and many have also been found in apparently unaffected individuals, thus raising questions regarding their pathogenic role in the disease. Deleterious effects on prokineticin signalling, however, have subsequently been shown in vitro for nearly all the missense mutations.67, 68 The finding, for given PROKR2 and PROK2 mutations, of both heterozygous and homozygous (or compound heterozygous) unrelated patients10, 69 is quite remarkable, and argues in favour of a digenic or oligogenic mode of inheritance in heterozygous patients. To date, digenic inheritance of KS has been shown in three such patients, who had monoallelic missense mutations both in PROKR2 and PROK2,67 FGFR1 (C Dodé, unpublished), or KAL1.10 It is tempting to speculate that the latter patient bears a hypomorphic KAL1 allele encoding a protein variant that would still retain some biological activity, whereas the vast majority of the KAL1 mutations reported so far lead to null alleles that are apparently sufficient to produce the abnormal phenotype in males. Other patients carrying heterozygous mutations in PROKR2, PROK2, or hypomorphic mutations in KAL1 are expected to carry additional mutations in other, as yet unknown, KS genes. Indeed, mutations in the five known genes together account for less than 30% of KS cases, indicating that other genes responsible for the disease remain to be discovered, some of which might also be involved in FGF signalling or prokineticin signalling. Notably, recent evidence indicates that oligogenic mode of inheritance may also apply to patients carrying mutations in FGFR1 or FGF8. Three patients carrying missense mutations in FGFR1 have indeed been found to also have a monoallelic or a biallelic mutation in FGF8,64 or a monoallelic mutation in PROKR2 (see above).
Genotype–phenotype correlation
For each genetic form of KS identified so far, the clinical heterogeneity of the disease within affected families clearly indicates that the manifestation of KS phenotypes is dependent on factors other than the mutated gene itself. These factors probably include epigenetic factors and modifier genes, both of which have not yet been identified. In addition, digenic or oligogenic inheritance presumably accounts in part for the long recognised incomplete penetrance of the disease. That said, some general features have emerged from clinical observations in the patients affected by the different genetic forms of KS. For instance, a greater variability in the degree of hypogonadism has been observed in patients carrying mutations in FGFR1, FGF8, PROKR2, or PROK2, than in KAL1 patients.7, 10, 65, 70, 71, 72 In particular, spontaneously fertile individuals carrying mutations in any of the four autosomal KS genes account for the transmission of the disease over several generations, whereas the X-linked form of KS is usually transmitted by the female carriers of KAL1 mutations, who are clinically unaffected. Among the variety of non-reproductive and non-olfactory disorders that affect a fraction of the KS patients, some have been reported for specific genetic forms of the disease. For instance, unilateral renal agenesis occurs in approximately 30% of KAL1 patients,31, 32 but has so far not been reported in patients with FGFR1, FGF8, PROKR2, or PROK2 mutations. On the other hand, the loss of nasal cartilage, external ear hypoplasia, and skeletal anomalies of the hands or feet, have only been reported in KAL2 patients.7, 13, 48 By contrast, hearing impairment is common to several genetic forms of KS,7, 8, 13, 23, 24, 29, 65 although it should be noted that the underlying defect (conductive, perceptive, or mixed) is likely to vary between different genetic forms. Palate defects should also be considered as one of these shared traits, even though the severity differs between KAL1 (high arched palate) and KAL2 (cleft palate). The cleft lip and/or palate may occur in as many as 25–30% of the KAL2 cases.7, 11, 12, 13, 65, 73 Lastly, bimanual synkinesis is highly prevalent in KAL1 (maybe >75% of the cases),17, 22 but seems to be much less common in KAL2.7, 12 Table 1 displays a comparison between KAL1 and KAL2 clinical features. Additional anomalies have so far not been reported in KS patients carrying mutations in PROKR2 or PROK2, with the notable exception of a severe sleep disorder and marked obesity in one patient,10 which could be related to the known function of prokineticin-2 signalling in behavioural circadian rhythms, including sleep–wake and ingestive behaviour.74 The prevalence of sleeping and eating disorders in KS patients, however, remains to be determined.
Table 1. A clinical comparison between KAL1 and KAL2 genetic forms of Kallmann syndrome.
| Genetic form | KAL1 | KAL2 |
|---|---|---|
| Gene (location) | KAL1 (Xp22.3) | FGFR1 (8p12) |
| Mode of transmission | X chromosome-linked | Autosomal dominant (incomplete penetrance) |
| Smell deficiency | Hyposmia to anosmia | None to anosmia |
| Hypogonadism | Usually severe | Highly variable |
| Non-reproductive and non-olfactory anomalies | ||
| Bimanual synkinesis | Yes (>75%) | Uncommon |
| Renal agenesis | Yes (30%) | Not reported |
| Cleft lip/palate | No, but high arched palate | Yes (25–30%) |
| Tooth agenesis | Yes | Yes (frequent?) |
| Hearing impairment | Yes (unknown frequency) | Yes (unknown frequency) |
| Other anomalies | Pes cavus, ptosis | Corpus callosum agenesis, external ear hypoplasia, absent nasal cartilage, hand/foot skeletal anomalies, iris coloboma |
Treatment of the hypogonadism
The treatment of hypogonadism in KS aims first to initiate virilisation or breast development, and second to develop fertility. Hormone replacement therapy, usually with testosterone for males and combined oestrogen and progesterone for females, is the treatment to stimulate the development of secondary sexual characteristics. For those desiring fertility, either gonadotropins or pulsatile GnRH can be used to obtain testicular growth and sperm production in males or ovulation in females. Both treatments restore fertility in a vast majority of affected individuals.75 It is still unknown whether transient hormone replacement therapy in affected male infants to simulate the postnatal surge in gonadotropins could have later impact on their sexual life and reproductive prognosis (see reference58).
Pathophysiology
Most phenotypic anomalies reported in KS may result from the developmental failures during the organogenesis period, between 4 and 10 embryonic weeks (see reference76 for review). The developmental disorder leading to the absence (or hypoplasia) of the olfactory bulbs and tracts, and to anosmia in KS is not completely understood. It may involve a failure of the terminal elongation or targeting of olfactory axons, a primary morphogenetic defect of the olfactory bulbs (at the end of the 6th embryonic week), and a later defect in axonal branching of the olfactory bulb output neurons. In the late 1980s, new light was shed on the mechanism of the GnRH deficiency underlying KS hypogonadism, with the discovery of a close topographic link between the peripheral olfactory system and neuroendocrine GnRH cells during the embryonic life. These cells undergo a migration, beginning in the 6th embryonic week, from the olfactory epithelium to the forebrain along the olfactory nerve pathway.77 In a human fetus carrying a chromosomal deletion at Xp22.3 that included KAL1, it was shown that GnRH cells had not migrated normally and had accumulated in the upper nasal region.78 There are presently no pathohistological data to verify that the embryonic migration of GnRH cells is arrested in individuals affected by other genetic forms of KS. In Prokr2 or Prok2 homozygous knockout mice, and mice carrying Fgfr1 or Fgf8 hypomorphic mutations in the homozygous state, however, the migration of these cells is disrupted too.14, 65, 79, 80 The mechanism of the putative defect of GnRH cell migration in KS is still conjectural. It could be either a consequence of the early degeneration of olfactory nerve and terminal nerve axons, which act as guiding cues, or a process directly affecting the GnRH cells themselves. Moreover, defects in GnRH cell fate specification, differentiation, axon elongation, or axon targeting to the hypothalamus median eminence may also contribute to the GnRH deficiency, at least in the KAL2 genetic form of the disease (see reference35, 80).
Unresolved questions
Although KS was identified as a hereditary disease more than 60 years ago, its genetics is still incompletely understood, including its much higher prevalence in males than in females. Among many unresolved genetic and clinical questions are the following:
What is the actual prevalence of KS, especially in females?
What is the KS phenotypic spectrum, especially with respect to non-reproductive non-olfactory additional disorders?
Is hereditary anosmia without apparent hypogonadism a clinical form of KS?
Where is the nosological frontier between KS and normosmic HH?
How many different disease genes are involved in KS, and how do they functionally interact in the development of olfactory and GnRH neuroendocrine systems (see reference81 for current hypotheses)?
What is the prevalence of the digenic/oligogenic mode of inheritance among KS patients? Indeed, answer to this question is a prerequisite to assess disease recurrence risk in the affected families.
Why is KS more frequent in males than in females?
Given that the X-linked recessive form does not account for the higher disease prevalence in males, could it be that females are to some extent protected against disease occurrence by physiologically higher levels of KAL1 expression during the embryonic life compared to males (because KAL1 partially escapes the X-chromosome inactivation process in humans62)?
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Maestre de San Juan A. Falta total de los nervios olfactorios con anosmía en un individuo en quien existia una atrofía congénita de los testículos y miembro viril. Siglo Medico. 1856;131:211. [Google Scholar]
- Kallmann FJ, Schoenfeld WA, Barrera SE. The genetic aspects of primary eunuchoidism. Am J Mental Deficiency. 1944;XLVIII:203–236. [Google Scholar]
- de Morsier G, Gauthier G. La dysplasie olfacto-génitale. Pathol Biol. 1963;11:1267–1272. [PubMed] [Google Scholar]
- Naftolin F, Harris GW, Bobrow M. Effect of purified luteinizing hormone releasing factor on normal and hypogonadotropic anosmic men. Nature. 1971;232:496–497. doi: 10.1038/232496a0. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Paulsen CA. Hypogonadotropic eunuchoidism. I. Clinical study of the mode of inheritance. J Clin Endocrinol Metab. 1972;36:47–54. doi: 10.1210/jcem-36-1-47. [DOI] [PubMed] [Google Scholar]
- Lieblich JM, Rogol AD, White BJ, Rosen SW. Syndrome of anosmia with hypogonadotropic hypogonadism (Kallmann syndrome) Am J Med. 1982;73:506–519. doi: 10.1016/0002-9343(82)90329-1. [DOI] [PubMed] [Google Scholar]
- Dodé C, Levilliers J, Dupont J-M, et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33:463–465. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]
- Massin N, Pêcheux C, Eloit C, et al. X chromosome-linked Kallmann syndrome: clinical heterogeneity in three siblings carrying an intragenic deletion of the KAL-1 gene. J Clin Endocrinol Metab. 2003;88:2003–2008. doi: 10.1210/jc.2002-021981. [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Acierno J, Meysing A, Dwyer A, Hayes F, Crowley W. Reversible Kallmann syndrome, delayed puberty, and isolated anosmia occurring in a single family with a mutation in the FGFR1 gene. J Clin Endocrinol Metab. 2005;90:1317–1322. doi: 10.1210/jc.2004-1361. [DOI] [PubMed] [Google Scholar]
- Dodé C, Teixeira L, Levilliers J, et al. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genetics. 2006;2:1648–1652. doi: 10.1371/journal.pgen.0020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitteloud N, Acierno JS, Jr, Meysing A, et al. Mutations in fibroblast growth factor receptor 1 cause both Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 2006;103:6281–6286. doi: 10.1073/pnas.0600962103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trarbach EB, Costa EM, Versiani B, et al. Novel fibroblast growth factor receptor 1 mutations in patients with congenital hypogonadotropic hypogonadism with and without anosmia. J Clin Endocrinol Metab. 2006;91:4006–4012. doi: 10.1210/jc.2005-2793. [DOI] [PubMed] [Google Scholar]
- Dodé C, Fouveaut C, Mortier G, et al. Novel FGFR1 sequence variants in Kallmann syndrome, and genetic evidence that the FGFR1c isoform is required in olfactory bulb and palate morphogenesis. Hum Mutat. 2007;28:97–98. doi: 10.1002/humu.9470. [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Zhang C, Pignatelli D, et al. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 2007;104:17447–17452. doi: 10.1073/pnas.0707173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanussen M, Sippell WG. Heterogeneity of Kallmann's syndrome. Clin Genet. 1985;28:106–111. doi: 10.1111/j.1399-0004.1985.tb00368.x. [DOI] [PubMed] [Google Scholar]
- Hipkin L, Casson I, Davis J. Identical twins discordant for Kallmann's syndrome. J Med Genet. 1990;27:198–199. doi: 10.1136/jmg.27.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton R, Duke VM, Robertson A, et al. Idiopathic gonadotrophin deficiency: genetic questions addressed through phenotypic characterization. Clin Endocrinol (Oxf) 2001;55:163–174. doi: 10.1046/j.1365-2265.2001.01277.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro RS, Vieira TC, Abucham J. Reversible Kallmann syndrome: report of the first case with a KAL1 mutation and literature review. Eur J Endocrinol. 2007;156:285–290. doi: 10.1530/eje.1.02342. [DOI] [PubMed] [Google Scholar]
- Raivio T, Falardeau J, Dwyer A, et al. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007;357:863–873. doi: 10.1056/NEJMoa066494. [DOI] [PubMed] [Google Scholar]
- Conrad B, Kriebel J, Hetzel WD. Hereditary bimanual synkinesis combined with hypogonadotropic hypogonadism and anosmia in four brothers. J Neurol. 1978;218:263–274. doi: 10.1007/BF00312882. [DOI] [PubMed] [Google Scholar]
- Schwankhaus JD, Currie J, Jaffe MJ, Rose SR, Sherins RJ. Neurologic findings in men with isolated hypogonadotropic hypogonadism. Neurology. 1989;39:223–226. doi: 10.1212/wnl.39.2.223. [DOI] [PubMed] [Google Scholar]
- Quinton R, Duke VM, de Zoysa PA, et al. The neuroradiology of Kallmann's syndrome: a genotypic and phenotypic analysis. J Clin Endocrinol Metab. 1996;81:3010–3017. doi: 10.1210/jcem.81.8.8768867. [DOI] [PubMed] [Google Scholar]
- Söderlund D, Canto P, Méndez J. Identification of three novel mutations in the KAL1 gene in patients with Kallmann syndrome. J Clin Endocrinol Metab. 2002;87:2589–2592. doi: 10.1210/jcem.87.6.8611. [DOI] [PubMed] [Google Scholar]
- Hardelin J-P, Levilliers J, Young J, et al. Xp22.3 deletions in isolated familial Kallmann's syndrome. J Clin Endocrinol Metab. 1993;76:827–831. doi: 10.1210/jcem.76.4.8473391. [DOI] [PubMed] [Google Scholar]
- Reardon W. Kallmann syndrome presenting as congenital ptosis in brothers. Clin Dysmorphol. 2007;16:207–208. doi: 10.1097/MCD.0b013e3280b10beb. [DOI] [PubMed] [Google Scholar]
- Kertzman C, Robinson DL, Sherins RJ, Schwankhaus JD, Mc Clurkin JW. Abnormalities in visual spatial attention in men with mirror movements associated with isolated hypogonadotropic hypogonadism. Neurology. 1990;40:1057–1063. doi: 10.1212/wnl.40.7.1057. [DOI] [PubMed] [Google Scholar]
- Hill J, Elliott C, Colquhoun I. Audiological, vestibular and radiological abnormalities in Kallmann's syndrome. J Laryngol Otol. 1992;106:530–534. doi: 10.1017/s0022215100120067. [DOI] [PubMed] [Google Scholar]
- Coatesworth AP, Woodhead CJ. Conductive hearing loss associated with Kallmann's syndrome. J Laryngol Otol. 2002;116:125–126. doi: 10.1258/0022215021909845. [DOI] [PubMed] [Google Scholar]
- Sato N, Katsumata N, Kagami M, et al. Clinical assessment and mutation analysis of Kallmann syndrome 1 (KAL1) and fibroblast growth factor receptor 1 (FGFR1, or KAL2) in five families and 18 sporadic patients. J Clin Endocrinol Metab. 2004;89:1079–1088. doi: 10.1210/jc.2003-030476. [DOI] [PubMed] [Google Scholar]
- Wegenke JD, Uehling DT, Wear JB, Jr, et al. Familial Kallmann syndrome with unilateral renal aplasia. Clin Genet. 1975;7:368–381. doi: 10.1111/j.1399-0004.1975.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Hardelin J-P, Levilliers J, Blanchard S, et al. Heterogeneity in the mutations responsible for X chromosome-linked Kallmann syndrome. Hum Mol Genet. 1993;2:373–377. doi: 10.1093/hmg/2.4.373. [DOI] [PubMed] [Google Scholar]
- Kirk J, Grant D, Besser G, et al. Unilateral renal aplasia in X-linked Kallmann's syndrome. Clin Genet. 1994;46:260–262. doi: 10.1111/j.1399-0004.1994.tb04238.x. [DOI] [PubMed] [Google Scholar]
- Molsted K, Kjaer I, Giwercman A, Versterhauge S, Skakkebaek N. Craniofacial morphology in patients with Kallmann's syndrome with and without cleft lip and palate. Cleft Palate-Craniofacial J. 1997;34:417–424. doi: 10.1597/1545-1569_1997_034_0417_cmipwk_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- de Zegher F, Lagae L, Declerck D, Vinckier F. Kallmann syndrome and delayed puberty associated with agenesis of lateral maxillary incisors. J Craniofac Genet Dev Biol. 1995;15:87–89. [PubMed] [Google Scholar]
- Tsai P-S, Gill J. Mechanisms of disease: insights into X-linked and autosomal-dominant Kallmann syndrome. Nat Clin Pract Endocrinol Metab. 2006;2:160–171. doi: 10.1038/ncpendmet0119. [DOI] [PubMed] [Google Scholar]
- Ballabio A, Andria G. Deletions and translocations involving the distal short arm of the human X chromosome: review and hypotheses. Hum Mol Genet. 1992;1:221–227. doi: 10.1093/hmg/1.4.221. [DOI] [PubMed] [Google Scholar]
- de Roux N, Young J, Misrahi M, et al. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med. 1997;337:1597–15602. doi: 10.1056/NEJM199711273372205. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Quinton R, Pearce S, et al. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007;117:457–463. doi: 10.1172/JCI29884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlaville D, Verloes A. CHARGE syndrome: an update. Eur J Hum Genet. 2007;15:389–399. doi: 10.1038/sj.ejhg.5201778. [DOI] [PubMed] [Google Scholar]
- Pinto G, Abadie V, Mesnage R, et al. CHARGE syndrome includes hypogonadotropic hypogonadism and abnormal olfactory bulb development. J Clin Endocrinol Metab. 2005;90:5621–5626. doi: 10.1210/jc.2004-2474. [DOI] [PubMed] [Google Scholar]
- Blustajn J, Kirsch CF, Panigrahy A, Netchine I. Olfactory anomalies in CHARGE syndrome: imaging findings of a potential major diagnostic criterion. AJNR Am J Neuroradiol. 2008;29:1266–1269. doi: 10.3174/ajnr.A1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez AB, Galindo A, Arensman FW, Van Dop C. Congenital heart disease associated with sporadic Kallmann syndrome. Am J Med Genet. 1993;46:551–554. doi: 10.1002/ajmg.1320460518. [DOI] [PubMed] [Google Scholar]
- Klein VR, Friedman JM, Brookshire GS, Brown OE, Edman CD. Kallmann syndrome associated with choanal atresia. Clin Genet. 1987;31:224–227. doi: 10.1111/j.1399-0004.1987.tb02800.x. [DOI] [PubMed] [Google Scholar]
- Ogata T, Fujiwara I, Ogawa E, Sato N, Udaka T, Kosaki K. Kallmann syndrome phenotype in a female patient with CHARGE syndrome and CHD7 mutation. Endocr J. 2006;53:741–743. doi: 10.1507/endocrj.k06-099. [DOI] [PubMed] [Google Scholar]
- Tellier AL, Cormier-Daire V, Abadie V, et al. CHARGE syndrome: report of 47 cases and review. Am J Med Genet. 1998;76:402–409. doi: 10.1002/(sici)1096-8628(19980413)76:5<402::aid-ajmg7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Zenaty D, Bretones P, Lambe C, et al. Paediatric phenotype of Kallmann syndrome due to mutations of fibroblast growth factor receptor 1 (FGFR1) Mol Cell Endocrinol. 2006;254–255:78–83. doi: 10.1016/j.mce.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Vissers LE, van Ravenswaaij CM, Admiraal R, et al. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36:955–957. doi: 10.1038/ng1407. [DOI] [PubMed] [Google Scholar]
- Lalani SR, Safiullah AM, Fernbach SD, et al. Spectrum of CHD7 mutations in 110 individuals with CHARGE syndrome and genotype–phenotype correlation. Am J Hum Genet. 2006;78:303–314. doi: 10.1086/500273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S, Gann P, Rogol A. Congenital anosmia:detection thresholds for seven odorant classes in hypogonadal and eugonadal patients. Ann Otol Rhinol Laryngol. 1979;88:288. doi: 10.1177/000348947908800223. [DOI] [PubMed] [Google Scholar]
- Doty R, Shaman P, Dann M. Development of the University of Pennsylvania Smell identification Test, a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Davidson TM, Murphy C. Rapid clinical evaluation of anosmia. The alcohol sniff test. Arch Otolaryngol Head Neck Surg. 1997;123:591–594. doi: 10.1001/archotol.1997.01900060033005. [DOI] [PubMed] [Google Scholar]
- Chalouhi C, Faulcon P, Le Bihan C, Hertz-Pannier L, Bonfils P, Abadie V. Olfactory evaluation in children: application to the CHARGE syndrome. Pediatrics. 2005;116:e81–e88. doi: 10.1542/peds.2004-1970. [DOI] [PubMed] [Google Scholar]
- Klingmüller D, Duwes W, Krahe T, Brecht G, Schweikert H-U. Magnetic resonance imaging of the brain in patients with anosmia and hypothalamic hypogonadism (Kallmann's syndrome) J Clin Endocrinol Metab. 1987;65:581–584. doi: 10.1210/jcem-65-3-581. [DOI] [PubMed] [Google Scholar]
- Whitcomb R, Crowley WJ. Male hypogonadotropic hypogonadism. Endocrinol Metab Clin North Am. 1993;22:125–143. [PubMed] [Google Scholar]
- Seminara SB, Hayes FJ, Crowley WF., Jr Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998;19:521–539. doi: 10.1210/edrv.19.5.0344. [DOI] [PubMed] [Google Scholar]
- Grumbach MM. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. J Clin Endocrinol Metab. 2005;90:3122–3127. doi: 10.1210/jc.2004-2465. [DOI] [PubMed] [Google Scholar]
- Truwit CL, Barkovich AJ, Grumbach MM, Martini JJ. MR imaging of Kallmann syndrome, a genetic disorder of neuronal migration affecting the olfactory and genital systems. AJNR Am J Neuroradiol. 1993;14:827–838. [PMC free article] [PubMed] [Google Scholar]
- Birnbacher R, Wandl-Vergesslich K, Frisch H. Diagnosis of X-recessive Kallmann syndrome in early infancy. Evidence of hypoplastic rhinencephalon. Eur J Pediatr. 1994;153:245–247. doi: 10.1007/BF01954511. [DOI] [PubMed] [Google Scholar]
- White BJ, Rogol AD, Brown KS, Lieblich JM, Rosen SW. The syndrome of anosmia with hypogonadotropic hypogonadism: a genetic study of 18 new families and a review. Am J Med Genet. 1983;15:417–435. doi: 10.1002/ajmg.1320150307. [DOI] [PubMed] [Google Scholar]
- Franco B, Guioli S, Pragliola A, et al. A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353:529–536. doi: 10.1038/353529a0. [DOI] [PubMed] [Google Scholar]
- Legouis R, Hardelin J-P, Levilliers J, et al. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell. 1991;67:423–435. doi: 10.1016/0092-8674(91)90193-3. [DOI] [PubMed] [Google Scholar]
- Hardelin J-P, Levilliers J, del Castillo I, et al. X chromosome-linked Kallmann syndrome: stop mutations validate the candidate gene. Proc Natl Acad Sci USA. 1992;89:8190–8194. doi: 10.1073/pnas.89.17.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falardeau J, Chung WC, Beenken A, et al. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118:2822–2831. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Ohyama K, Fukami M, Okada M, Ogata T. Kallmann syndrome: somatic and germline mutations of the fibroblast growth factor receptor 1 gene in a mother and the son. J Clin Endocrinol Metab. 2006;91:1415–1418. doi: 10.1210/jc.2005-2266. [DOI] [PubMed] [Google Scholar]
- Cole LW, Sidis Y, Zhang C, et al. Mutations in prokineticin 2 (PROK2) and PROK2 receptor 2 (PROKR2) in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab. 2008;93:3551–3559. doi: 10.1210/jc.2007-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier C, Dodé C, Fabre L, et al. PROKR2 missense mutations associated with Kallmann syndrome impair receptor signalling-activity Hum Mol Genet 2008[Epub ahead of print]. PMID 18826963. [DOI] [PMC free article] [PubMed]
- Leroy C, Fouveaut C, Leclercq S, et al. Biallelic mutations in the prokineticin-2 gene in two sporadic cases of Kallmann syndrome. Eur J Hum Genet. 2008;16:865–868. doi: 10.1038/ejhg.2008.15. [DOI] [PubMed] [Google Scholar]
- Oliveira LM, Seminara SB, Beranova M, et al. The importance of autosomal genes in Kallmann syndrome: genotype-phenotype correlations and neuroendocrine characteristics. J Clin Endocrinol Metab. 2001;86:1532–1538. doi: 10.1210/jcem.86.4.7420. [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Meysing A, Quinton R, et al. Mutations in fibroblast growth factor receptor 1 cause Kallmann syndrome with a wide spectrum of reproductive phenotypes. Mol Cell Endocrinol. 2006;254–255:60–69. doi: 10.1016/j.mce.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Salenave S, Chanson P, Bry H, et al. Kallmann′s syndrome: a comparison of the reproductive phenotypes in men carrying KAL1 and FGFR1/KAL2 mutations. J Clin Endocrinol Metab. 2008;93:758–763. doi: 10.1210/jc.2007-1168. [DOI] [PubMed] [Google Scholar]
- Albuisson J, Pêcheux C, Carel J-C, et al. Kallmann syndrome: 14 novel mutations in KAL1 and FGFR1 (KAL2) Hum Mutat. 2005;25:98–99. doi: 10.1002/humu.9298. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Cheng MY. Prokineticin 2 and circadian clock output. FEBS J. 2005;272:5703–5709. doi: 10.1111/j.1742-4658.2005.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchter D, Behre HM, Kliesch S, Nieschlag E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol. 1998;139:298–303. doi: 10.1530/eje.0.1390298. [DOI] [PubMed] [Google Scholar]
- Hardelin J-P, Dodé C.KAL1, FGFR1, PROKR2, PROK2, and Kallmann syndromein: Epstein C, Erickson R, Wynshaw-Boris A (eds): Inborn errors of development New York: Oxford University Press; 2008. chapter 47, pp482–490. [Google Scholar]
- Schwanzel-Fukuda M, Crossin KL, Pfaff DW, Bouloux PMG, Hardelin J-P, Petit C. Migration of luteinizing hormone-releasing hormone (LHRH) neurons in early human embryos. J Comp Neurol. 1996;366:547–557. doi: 10.1002/(SICI)1096-9861(19960311)366:3<547::AID-CNE12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Mol Brain Res. 1989;6:311–326. doi: 10.1016/0169-328x(89)90076-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Yamazaki C, Masumoto KH, et al. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA. 2006;103:4140–4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WC, Moyle SS, Tsai PS. Fibroblast growth factor 8 signaling through Fgf receptor 1 is required for the emergence of gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:4997–5003. doi: 10.1210/en.2007-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardelin J-P, Dodé C. The complex genetics of Kallmann syndrome: KAL1, FGFR1, FGF8, PROKR2, PROK2, et al. Sex Dev. 2008;2:181–193. doi: 10.1159/000152034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.