Abstract
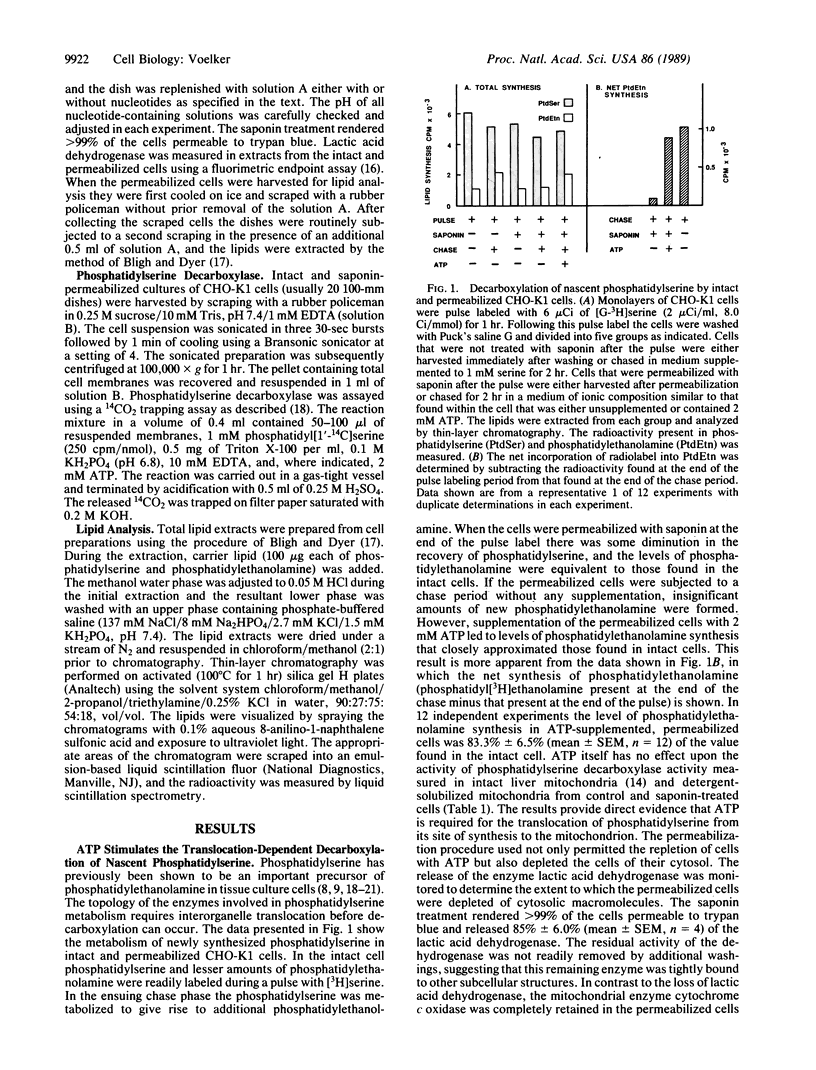
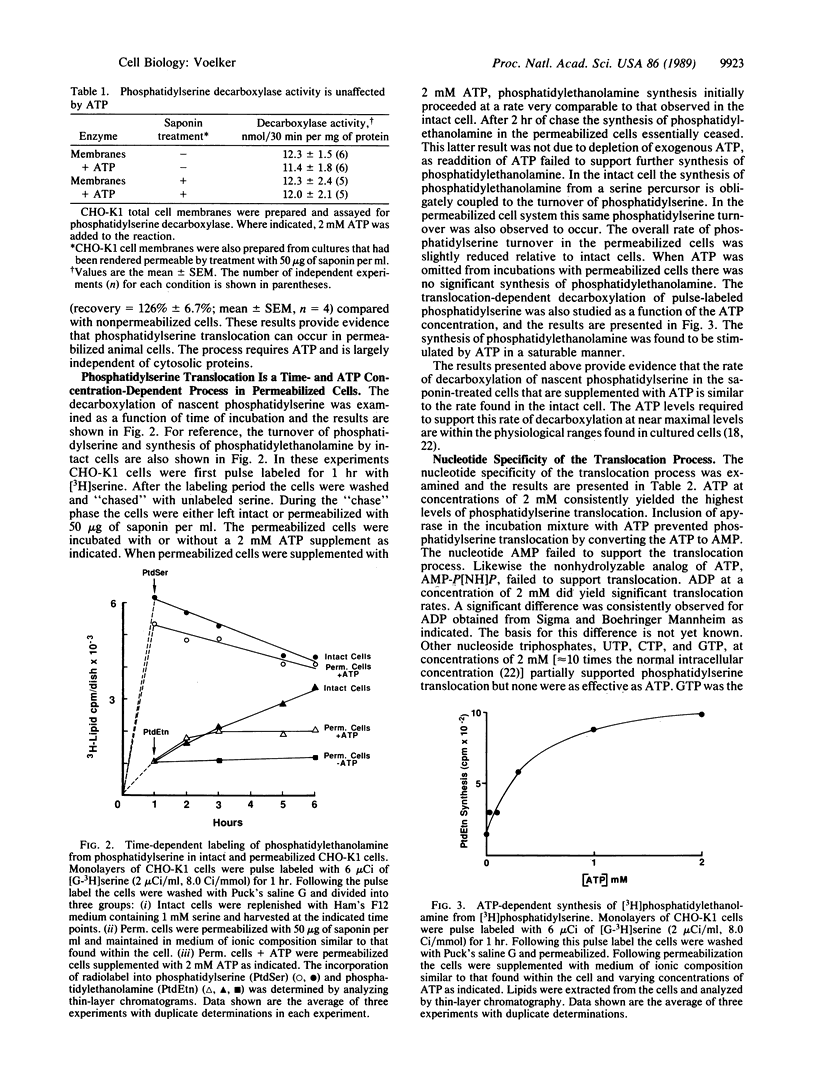
Chinese hamster ovary (CHO-K1) cells were pulse labeled with [3H]serine, and the synthesis of phosphatidyl[3H]ethanolamine from phosphatidyl[3H]serine during the subsequent chase was used as a measure of lipid translocation to the mitochondria. When the CHO-K1 cells were pulse labeled and subsequently permeabilized with 50 micrograms of saponin per ml, there was no significant turnover of nascent phosphatidyl[3H]serine to form phosphatidyl[3H]ethanolamine during an ensuing chase. Saponin treatment rendered greater than 99% of the cells permeable as judged by trypan blue exclusion and depleted them of 85% of their complement of cytosolic proteins as determined by residual lactic acid dehydrogenase activity. Supplementation of the permeabilized cells with 2 mM ATP resulted in significant phosphatidyl[3H]ethanolamine synthesis (83% of that found in intact cells) from phosphatidyl[3H]serine during a subsequent 2-hr chase. Phosphatidyl[3H]ethanolamine synthesis essentially ceased after 2 hr in the permeabilized cells. The translocation-dependent synthesis of phosphatidyl[3H]ethanolamine was a saturable process with respect to ATP concentration in permeabilized cells. The conversion of phosphatidyl[3H]serine to phosphatidyl[3H]ethanolamine did not occur in saponin-treated cultures supplemented with 2 mM AMP, 2 mM 5'-adenylyl imidodiphosphate, or apyrase (2.5 units/ml) plus 2 mM ATP. ATP was the most effective nucleotide, but the addition of GTP, CTP, UTP, and ADP also supported the translocation-dependent synthesis of phosphatidyl[3H]ethanolamine albeit to a lesser extent. These data provide evidence that the interorganelle translocation of phosphatidylserine requires ATP and is largely independent of soluble cytosolic proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chlapowski F. J., Band R. N. Assembly of lipids into membranes in Acanthamoeba palestinensis. II. The origin and fate of glycerol- 3 H--labeled phospholipids of cellular membranes. J Cell Biol. 1971 Sep;50(3):634–651. doi: 10.1083/jcb.50.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. A., Kennedy E. P. Intracellular sites of lipid synthesis and the biogenesis of mitochondria. J Lipid Res. 1972 Mar;13(2):263–267. [PubMed] [Google Scholar]
- Dyatlovitskaya E. V., Timofeeva N. G., Bergelson L. D. A universal lipid exchange protein from rat hepatoma. Eur J Biochem. 1978 Jan 16;82(2):463–471. doi: 10.1111/j.1432-1033.1978.tb12040.x. [DOI] [PubMed] [Google Scholar]
- Fanestil D. D., Barrows C. H., Jr Aging in the rotifer. J Gerontol. 1965 Oct;20(4):462–469. [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Jelsema C. L., Morré D. J. Distribution of phospholipid biosynthetic enzymes among cell components of rat liver. J Biol Chem. 1978 Nov 10;253(21):7960–7971. [PubMed] [Google Scholar]
- Kaplan M. R., Simoni R. D. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane. J Cell Biol. 1985 Aug;101(2):446–453. doi: 10.1083/jcb.101.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge O., Nishijima M., Akamatsu Y. Isolation of a somatic-cell mutant defective in phosphatidylserine biosynthesis. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1926–1930. doi: 10.1073/pnas.82.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge O., Nishijima M., Akamatsu Y. Phosphatidylserine biosynthesis in cultured Chinese hamster ovary cells. II. Isolation and characterization of phosphatidylserine auxotrophs. J Biol Chem. 1986 May 5;261(13):5790–5794. [PubMed] [Google Scholar]
- Megli F. M., De Lisi A., van Amerongen A., Wirtz K. W., Quagliariello E. Nonspecific lipid transfer protein (sterol carrier protein 2) is bound to rat liver mitochondria: its role in spontaneous intermembrane phospholipid transfer. Biochim Biophys Acta. 1986 Oct 23;861(3):463–470. doi: 10.1016/0005-2736(86)90455-4. [DOI] [PubMed] [Google Scholar]
- Miller M. A., Kent C. Characterization of the pathways for phosphatidylethanolamine biosynthesis in Chinese hamster ovary mutant and parental cell lines. J Biol Chem. 1986 Jul 25;261(21):9753–9761. [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Poorthuis B. J., van der Krift T. P., Teerlink T., Akeroyd R., Hostetler K. Y., Wirtz K. W. Phospholipid transfer activities in Morris hepatomas and the specific contribution of the phosphatidylcholine exchange protein. Biochim Biophys Acta. 1980 Aug 4;600(2):376–386. doi: 10.1016/0005-2736(80)90441-1. [DOI] [PubMed] [Google Scholar]
- Post M., Batenburg J. J., Schuurmans E. A., van Golde L. M. Phospholipid-transfer activity in type II cells isolated from adult rat lung. Biochim Biophys Acta. 1980 Nov 7;620(2):317–321. doi: 10.1016/0005-2760(80)90212-x. [DOI] [PubMed] [Google Scholar]
- Sleight R. G., Pagano R. E. Transport of a fluorescent phosphatidylcholine analog from the plasma membrane to the Golgi apparatus. J Cell Biol. 1984 Aug;99(2):742–751. doi: 10.1083/jcb.99.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Chueh S. H., Noel M. W., Gill D. L. Influence of inositol 1,4,5-trisphosphate and guanine nucleotides on intracellular calcium release within the N1E-115 neuronal cell line. J Biol Chem. 1986 Mar 5;261(7):3184–3192. [PubMed] [Google Scholar]
- Vance D. E., Trip E. M., Paddon H. B. Poliovirus increases phosphatidylcholine biosynthesis in HeLa cells by stimulation of the rate-limiting reaction catalyzed by CTP: phosphocholine cytidylyltransferase. J Biol Chem. 1980 Feb 10;255(3):1064–1069. [PubMed] [Google Scholar]
- Vance J. E., Vance D. E. Does rat liver Golgi have the capacity to synthesize phospholipids for lipoprotein secretion? J Biol Chem. 1988 Apr 25;263(12):5898–5909. [PubMed] [Google Scholar]
- Voelker D. R. Disruption of phosphatidylserine translocation to the mitochondria in baby hamster kidney cells. J Biol Chem. 1985 Nov 25;260(27):14671–14676. [PubMed] [Google Scholar]
- Voelker D. R., Frazier J. L. Isolation and characterization of a Chinese hamster ovary cell line requiring ethanolamine or phosphatidylserine for growth and exhibiting defective phosphatidylserine synthase activity. J Biol Chem. 1986 Jan 25;261(3):1002–1008. [PubMed] [Google Scholar]
- Voelker D. R. Phosphatidylserine functions as the major precursor of phosphatidylethanolamine in cultured BHK-21 cells. Proc Natl Acad Sci U S A. 1984 May;81(9):2669–2673. doi: 10.1073/pnas.81.9.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker D. R. Reconstitution of phosphatidylserine import into rat liver mitochondria. J Biol Chem. 1989 May 15;264(14):8019–8025. [PubMed] [Google Scholar]
- Wirtz K. W., Jolles J., Westerman J., Neys F. Phospholipid exchange proteins in synaptosome and myelin fraction from rat brain. Nature. 1976 Mar 25;260(5549):354–355. doi: 10.1038/260354a0. [DOI] [PubMed] [Google Scholar]
- Wirtz K. W., Zilversmit D. B. Exchange of phospholipids between liver mitochondria and microsomes in vitro. J Biol Chem. 1968 Jul 10;243(13):3596–3602. [PubMed] [Google Scholar]
- Yaffe M. P., Kennedy E. P. Intracellular phospholipid movement and the role of phospholipid transfer proteins in animal cells. Biochemistry. 1983 Mar 15;22(6):1497–1507. doi: 10.1021/bi00275a026. [DOI] [PubMed] [Google Scholar]
- van Amerongen A., van Noort M., van Beckhoven J. R., Rommerts F. F., Orly J., Wirtz K. W. The subcellular distribution of the nonspecific lipid transfer protein (sterol carrier protein 2) in rat liver and adrenal gland. Biochim Biophys Acta. 1989 Feb 20;1001(3):243–248. doi: 10.1016/0005-2760(89)90106-9. [DOI] [PubMed] [Google Scholar]
- van Golde L. M., Raben J., Batenburg J. J., Fleischer B., Zambrano F., Fleischer S. Biosynthesis of lipids in Golgi complex and other subcellular fractions from rat liver. Biochim Biophys Acta. 1974 Aug 22;360(2):179–192. doi: 10.1016/0005-2760(74)90168-4. [DOI] [PubMed] [Google Scholar]



