Abstract
Atrial septal defect (ASD) is an incomplete septation of atria in human heart causing circulatory problems. Its frequency is estimated at one per 10 000. Actions of numerous genes have been linked to heart development. However, no single gene defect causing ASD has yet been identified. Incomplete heart septation similar to ASD was reported in transgenic mice with both inactive alleles of gene encoding mammalian zinc metalloprotease a mammalian tolloid-like 1 (tll1). Here, we have screened 19 ASD patients and 15 healthy age-matched individuals for mutations in TLL1 gene. All 22 exons were analyzed exon by exon for heteroduplex formation. Subsequently, DNA fragments forming heteroduplexes were sequenced. In four nonrelated patients, three missense mutations in coding sequence, and one single base change in the 5′UTR have been detected. Two mutations (Met182Leu, and Ala238Val) were detected in ASD patients with the same clinical phenotype. As the second mutation locates immediately upstream of the catalytic zinc-binding signature, it might change the enzyme substrate specificity. The third change, Leu627Val in the CUB3 domain, has been found in an ASD patient with interatrial septum aneurysm in addition to ASD. The CUB3 domain is important for substrate-specific recognition. In the remaining 15 patients as well as in 15 reference samples numerous base substitutions, deletions, and insertions have been detected, but no mutations changing the coding sequence have been found. Lack of mutations in relation to ASD of these patients could possibly be because of genetic heterogeneity of the syndrome.
Keywords: atrial septal defect, DNA sequencing, gene mutations, heart defects, heteroduplex analysis, mammalian tolloid-like 1
Introduction
Congenital heart defects in general are related to aberrant development of the circulation system in a fetus. The defects might result either from factors damaging the fetus during development or they can be of genetic background. Some environmental factors, drugs, alcohol abuse, and infections contracted by the mother before, or during pregnancy (eg, rubella) may alter heart development.1, 2, 3, 4, 5, 6 However, environmental and maternal factors have been linked to approximately 2% of congenital heart defects.7 In a significant number of heart abnormalities coexistence of a few anomalies has been shown in different parts of the circulation system indicating genetic factors.1, 8
Chromosomal anomalies and single gene defects, however, could be attributed to the background of 8% of congenital heart defects.7, 9, 10, 11 Thus, the background of most of the congenital heart defects is still poorly understood.
The heart itself and septation of the large vessels begin at the fourth week of fetal development, but the process is not completed until shortly after birth. Septation anomalies are linked to abnormal development of endocardial cushions. They are necessary for completeness of interatrial as well as interventrical septa. Incomplete interatrial septum is a common feature of such genetic disorders as Noonan, Turner, and Holt-Oram syndromes.10, 11, 12
The condition studied here is called atrial septal defect (ASD) and has some features common with the other just listed heart-related syndromes. Currently, the defects related to ASD are corrected during puberty. However, in the past, when it was untreated, the patients often suffered from numerous severe cardiovascular and pulmonary complications.
In recent years, results of research with the use of transgenic mice models revealed that a gene named tolloid-like 1 (tll1) is critical for septa development in the mouse heart.13 The mice lacking active TLL1 died at mid-gestation because of severe blood circulation failure. The tll1 gene product is a metalloprotease with domain structure identical to one of the alternatively spliced variants of the bmp-1 gene product, mammalian tolloid (mTLD). The mTLD was detected in mammals and some other invertebrates.14, 15, 16, 17, 18 The homologous protein BMP-1 was originally reported by Wozney et al19 in a context of ectopic bone formation in rats when protein extracts from demineralized bovine bones were used.20 Subsequently, based on protein domain structure it was classified in the family of astacin, a group of metalloproteases involved in embryo development of different species.17, 21, 22, 23 It is also commonly accepted that BMP-1 is important for numerous physiological activities acting in seemingly unrelated processes, such as extracellular matrix quality control involving formation and activation of numerous factors that act in the regulation of different genetic programs.17, 18, 19, 20, 21, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32
An investigation of different mouse model with ventrical spetum defect (VSD) but with active tll1 gene indicated that a different mechanism must be involved, possibly related to abnormal cell proliferation and differentiation.33, 34 Developmental defects of tll1−/− embryos seemed to be exclusively restricted to the heart.13 The comparison of the defects with expression of the tll1 revealed, in normal mice embryo, its elevated expression levels during heart septation. Thus, it is possible that the expression of TLL1 in humans could also be critical for correct heart positioning and its proper septation.
Here, we have tested 19 adult individuals with clinically confirmed ASD by karyotyping and screening of the TLL1 gene for mutations by heteroduplex detection followed by DNA sequencing.
Materials and methods
Patients
Hospital records of more than 100 cardiological patients have been reviewed for ASD cases. Twenty individuals have been identified who fulfill the requirements of this project. All of them were informed about procedures related to the study. The procedures were conducted in accordance with the Institutional Guidelines and Regulations and with the Guidelines for the Conduct of Research Involving Human Subjects approved by the appropriate institutional review board (IRB) Bioethical Committee of the Silesian Medical University in Katowice, Poland, No. L.dz.NN-013-339/03. In addition, appropriate informed consent was obtained from the human volunteers.
Finally, 19 individuals agreed to participate in the study and they all have been surveyed for medical and family health history. On the basis of the survey's results and medical records they were divided into three groups (Table 1).
Table 1. Classification of ASD patients based on their individual clinical symptoms.
| Patient number | Sex (F/M) | Patient's age (years) | Additional defects |
|---|---|---|---|
| Group 1: ASD without additional defects | |||
| 3 | M | 51 | ND |
| 5 | M | 55 | ND |
| 7 | M | 71 | ND |
| 10a | M | 35 | ND |
| 11 | M | 52 | ND |
| 15 | F | 57 | ND |
| 18 | F | 52 | ND |
| Group 2: ASD with diagnosed aneurysm of interatrial septum and other defects | |||
| 1 | M | 70 | Aneurysm, heart attack, cerebral stroke, atrial fibrillation, and cardiac pacemaker implantation |
| 4 | M | 55 | Heart attack |
| 6 | M | 45 | Hypertrophic cardiomyopathy |
| 9 | M | 72 | Aneurysm of interatrial septum sinus bradycardia |
| 12 | F | 58 | Asymptomatic aneurysm of interatrial septum |
| 13 | F | 48 | Aneurysm of interatrial septum |
| 17 | F | 48 | Asymptomatic aneurysm of interatrial septum |
| 19 | F | 62 | Artificial mitral valve implantation |
| Group 3: ASD with irregular heart beating rhythm | |||
| 2 | M | 52 | Atrial fibrillation, cardiac pacemaker implantation |
| 8 | M | 51 | Atrial fibrillation, cardiac pacemaker implantation |
| 14 | F | 56 | Cardiac pacemaker implantation |
| 16 | F | 67 | Atrial fibrillation |
ASD, atrial septal defect; F, female; M, male; ND, Not determined.
This patient was only the one who was classified with ASD type I. All the other patients had ASD type II.
As a reference, blood samples from 15 age-matching volunteers (11 females and 4 males) were used to isolate DNA for genetic analyses. None of the volunteers have had inherited developmental heart conditions.
Samples collection and handling
From each patient two blood samples were drawn. One 3 ml sample was mixed with heparin and used for cytogenetic diagnosis to exclude possible chromosomal anomalies. The methods for lymphocytes isolation and preparation for karyotyping were the classical methods described before.35, 36 Slides were examined with a Zeiss Axioskop microscope. Images were obtained using a Sony ExwaveHAD digital camera and MultiScanBase software (Computer Scanning System Ltd., PL) with the karyotype option, enabling manual karyotyping. For each patient, a total of 15 metaphases in each of five prepared karyograms were examined to determine the number of chromosomes.
The second sample of blood (5.0 ml) was mixed with EDTA and used for the isolation of genomic DNA.
PCR conditions
The DNA for mutation detection was isolated with the use of commercial blood mini-kit (A&A Biotechnology, Gdansk, Poland) according to supplier instructions. The quality of purified genomic DNA was verified by spectrometry at A260/280 and agarose gel electrophoresis.
Fragments of the TLL1 gene were amplified using pairs of primers designed to complement intronic sequences flanking coding sequences (Table 2). The conditions for amplification PCR were as follows: step (1) denaturation at 95°C for 4 min, step (2) denaturation at 95°C for 30 s, step (3) annealing at the appropriate primer pair melting temperature (Tm) (for details see Table 2) for 30 s, step (4) elongation at 72°C for 1 min per each 1250 bp of the amplified DNA fragment. The procedure was repeated 34 times from steps (2) to (4). Following the last annealing, the elongation time at 72°C was extended up to 7 min. The PCR products were purified using method provided in the sequencing manual by Applied Biosystems. The DNA concentration was determined spectrophotometrically at A260. The reference DNA was isolated from the blood of healthy individuals. The DNA reference fragments were obtained with the corresponding primers under the conditions used for patients' samples and subjected to heteroduplex analysis and DNA sequencing, when necessary except for the exons with mutations detected in patients that were sequenced entirely.
Table 2. PCR primers and conditions used for amplification and sequencing of the TLL1 gene.
| Exon number | Primers positions from–to | Product length (bp) | Primer sequence (length in bp) | Primer annealing temperature (°C) |
|---|---|---|---|---|
| 1 | 305–1626 | 1322 | CACgTACATCATCTATTAATTgCTgTTTgC (30) TCCCACTggCACAgAACTCTAgTCAACTg (29) | 58 |
| Int. 1 | 644–1626 | 983 | TgCAggCTTTTAAggTCTCgCggCgTAg (28) TCCCACTggCACAgAACTCTAgTCAACTg (29) | 62 |
| 2 | 116 547–118 357 | 1811 | ggTAgCTAATATTTgTCCTgTAAC (24) CTCTACTgTCCAgAATgCgTTgTC (24) | 60 |
| 3 | 119 808–120 855 | 1048 | gCAgAACAACTTgCTgTCATTCTATgTAg (29) ATgTACTTCAAgCATTTAATTAgTAATCCATC (32) | 52 |
| 4 and 5 | 121 553–122 696 | 1144 | gCTTTTgTTCAAgTATgTAATATAACTgAA (29) ACTgTgATCAAAgAATACACATACCTAgTAT (31) | 51 |
| 6 | 130 574–131 341 | 768 | ggATACCAgAAACTAgTCATATgTAgTAAT (30) CCTggTATAACACAgATTCCTATggTA (27) | 52 |
| 7 | 135 107–135 828 | 722 | CAATATgATggTAgAgTgAAgACAgATgC (29) ACACAgTATAAAgCACAAgTCgCCTAgATT (30) | 65 (−0.5°C per cycle) |
| 8 | 141 561–142 212 | 652 | GAATgTCATCATTgAgTTTATACTAg (26) ATCATgCAACTATTTAATTAATgACTATCA (30) | 50 |
| 9 | 152 432–153 304 | 873 | ATTTCTCTTATAATCTTATTCggTgATTCAC (31) TCTAACCTCTTCATATggTCgTCTAg (26) | 51 |
| 10 | 166 465–167 014 | 550 | ACTTCTCCTgACATTgTAAAACAgTTA (27) CCTAgTATgTACCTCATAAgTTAAgCTAg (29) | 50 |
| 11 | 169 104–169 695 | 592 | CATCTTACTTCTACATATggTAACAATA (28) gATATTATTACATCACTgATACTAgACAA (29) | 50 |
| 12 | 170 467–171 296 | 830 | CTACATTACAACCAgTCAACTTTgAT (26) CTgACCCgTCATCTAATAACACAAC (25) | 58 |
| 13 | 182 254–183 708 | 1455 | ggATATTACAgTgTACgTTCATgAgAAACA (30) gACAggATACTCCATAATCTCACTCTTCA (29) | 51 |
| 14 | 184 178–184 802 | 625 | gCAgCAACATgAATTTgTATACgTTATCCA (30) ATCAgCTgTgACTgTgAATTgAgTCTTACT (30) | 52 |
| 15 | 187 056–187 662 | 607 | TCTAAAgATAAgAAAACTgAgACCTAgA (28) TgAgCAgTAAGACAAAgAAAgAATACAATg (30) | 50 |
| 16 | 192 768–194 033 | 1266 | TggACAAgCTATATAAAAATCTCTgAACT (29) ACAAACCCTAgACATTgTAAATCTCAT (27) | 58 |
| 17 | 201 974–202 547 | 574 | gAggATTACAAgTTATTATCgTAgCg (26) CTAATgCCATCTATgTgCgAAgTAC (25) | 53 |
| 18 | 204 748–205 588 | 841 | ATTCAAgTgTATATTTAACTAAgAgAgA (28) CAggATAAgAAACCCTAgAggAgAACAACg (30) | 50 |
| 19 | 218 070–218 860 | 791 | AggATAATgTTCAACgggTTCTATgAT (27) CAAAgATCTTTAggTAggATgCTgTATggT (30) | 50 |
| 20 | 226 477–227 111 | 635 | CCAgTTgTggAAAACATATCTgACg (25) gAAATTATACCTACTgATTTCATCggT (27) | 58 |
| 21/22 | 227 995–231 411 | 3417 | gACAAgTATTCCCAAACATgCgTTAC (26) ACTgTgACATATTAAAATgAgCgACA (26) | 57 |
| Int. 21 | 227 995–229 978 | 1984 | gACAAgTATTCCCAAACATgCgTTAC (26) gTTgTATgCTCATggACAggTAACAgAC (28) | 55 |
Int., primer for starting sequencing PCR inside of exons 1 or 21.
Heteroduplex formation and analysis
Equal amounts (200 ng) of each, the reference and corresponding patients' DNA fragments, in 4 μl were mixed in the final volume of 10 μl supplemented with one-tenth volume of 0.5 KCl, and nuclease-free water when needed. The samples were handled according to the protocols recommended by the Transgenomic Inc., as follows: melting of the DNA at 95°C for 2 min and then cooling the mixture from 95 to 85°C at the rate 2.0°C per second and from 85 to 25°C at the rate 0.5°C per second. Subsequently, the mixture was subjected to the enzymic assay using Surveyor kit (Transgenomic Inc.). Briefly, the 400 ng of hybridized DNA in the volume 4–15 μl was mixed with 0.5 μl of Surveyor Nuclease S and 0.5 μl Surveyor Enhancer, and water, when needed, and incubated at 42°C for 40 min. The cleavage was terminated by mixing the sample with one-tenth volume of the Stop Solution supplied by the manufacturer in the assay kit. To analyze the Surveyor Nuclease S digestion products by agarose gel electrophoresis one-sixth volume of a 6X loading dye buffer (10 m Tris–HCl (pH 8.0), 10 m EDTA (pH 8.0), 50% (w/v) sucrose, 0.15% (w/v) bromophenol blue) was added to each sample. For each digestion/loading dye mixture, the entire volume of sample was loaded onto an agarose gel of an appropriate percentage (1.5–1.7%) and separated electrophoretically. The mixture of λ DNA cleaved with HindIII and φX cleaved with HaeIII (Finnzymes) was used as the DNA length marker. The gels were developed at 5 V/cm until the bromophenol blue had reached two-thirds of the length of the gel. To visualize the bands, an illumination of the gel using a UV transilluminator (250–300 nm) was performed and the pictures were taken.
DNA sequencing
Samples revealing heteroduplex formation with the corresponding fragment of reference DNA were subjected to DNA sequencing.37 Sequencing PCR was conducted using reagents supplied by Applied Biosystems under conditions recommended by the manufacturer. Briefly, the PCR products were purified by ethanol precipitation. The precipitated DNA was solubilized in the Hi-Di Formamide (Applied Biosystems) denatured at 95°C for 5 min, cooled and analyzed using ABI PRISM 310 Genetic Analyzer (Applied Biosystems). The sequence obtained was verified using the ChromasPro software. Sequence alignment was obtained with the use of MegAlign software in DNA-Star package v. 5.0, Lasergene. The reference sequence for TLL1 used here was AC097502.3.1.168254 (GenBank).
Results
As no chromosomal anomalies were detected by G-banding karyotype analysis in any of the ASD patients, further analyses focused on heteroduplex detection and subsequent DNA sequencing.
Heteroduplex formation frequency
All amplified PCR fragments were subjected to the DNA heteroduplex formation analysis. In two fragments containing exons 3 and 11, no heteroduplex formation was detected in any of the ASD individuals as well as in all 15 reference samples.
In all patients, mismatches were detected in gene fragments containing exons 1, 5, 12, 14, 19, and 20. The DNA fragments containing exons 2, 4, 6–10, 13, 15–18, 21, and 22 revealed various changes regarding the number of formed heteroduplexes as well as the number of mismatches in a single fragment. However, in 122 (34%) heteroduplexes found in patients, as well as in all heteroduplexes detected in reference samples, their sequencing excluded mismatches, indicating that the heteroduplex detection method also generated false-positive results. The remaining 66% of the heteroduplexes detected in patients resulted from changes located mostly in the intronic sequences. Some changes were located within the distance of 50 bp from the putative RNA splicing sites. Finally, 30 changes in the introns were confirmed by sequencing. They were substitutions: A to G (13), G to A (14), T to G (1), A to T (1), and C to T (1). The remaining changes detected in the heteroduplexes could not be verified by sequencing, as they were probably located in regions out of readable sequences.
Potential polymorphic changes in ASD patients
In the DNA gene fragment containing the exon 19 in six patients from group 1, in three patients from group 2, and in two patients from group 3 as well as in control sample, a polymorphic change A to G at the position 218 697 was detected. Another polymorphic change of T to C at position 218 734 of the same fragment was found in four patients from group 1, in three individuals from group 2, in three patients from group 3, and in the control sample. In addition, a polymorphic change in two patients, one from group 1 and the other one from group 2 as well as in the reference sample was identified at the position 218 441 (T to A). There was also a single base deletion (T del. 218 443) in four patients from group 1, in two individuals from group 2, and in two patients from group 3. Double-base insertion (TA ins. 218 328–9) has been detected in four patients from group 1, in two patients from group 2, and in two patients from group 3.
Mutations changing sense of codons
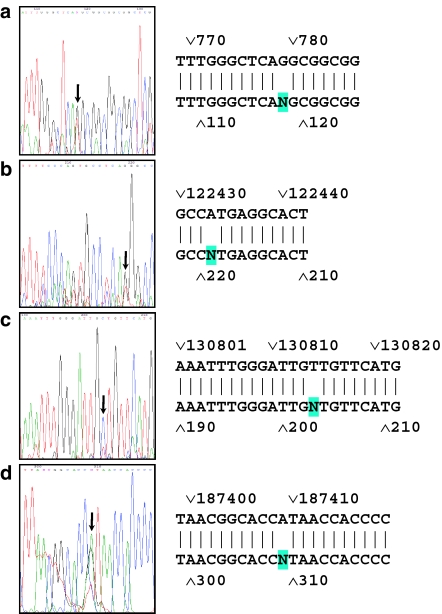
In all examined exons 4 changes detected by heteroduplex analysis were confirmed by DNA sequencing (Figure 1). The DNA fragment containing the exon 1 (305–1625) from one patient from group 2 revealed one mismatch (G/T) in the 5′UTR at position 779 (Figure 1a). The change, however, is not located within the Kozak consensus sequence and it was earlier annotated as SNP (GenBank – AC097502.3.1.168254).
Figure 1.
Heterozygous mutations detected in ASD patients. (a) A change G:G/T at position 779 detected in patient no. 17 from group 2 with ASD, aneurysm of interatrial septum and other defects. (b) A change A:A/C at position 122 433 (1191 in cDNA) detected in patient no. 10 from group 1 with ASD symptoms alone. In the presented case, the sequence was obtained with the use of reverse primer to sequence the fragment starting at the position 121 553 (exons 4 and 5). (c) A change T:C at position 130 814 (1360 in cDNA) detected in the patient no. 1 from group 2 with ASD, aneurysm of intersinal septum and other defects. (d) A change A:A/G at position 187 409 (1885 in cDNA) detected in the patient no. 9 from group 2 with ASD, aneurysm of intersinal septum and other defects. Arrow in each panel indicates the detected changes.
In the fragment containing exon 5, a heterozygous base substitution A:C at position 122 433 (Figure 1b) (1191 in the cDNA; NM_012464) changing a codon for Met to codon for Leu at position 182 was detected. Another mutation was detected in the fragment containing exon 6 in one patient from group 2. It was a change from T to C at position 130 814 changing valine 238 to alanine (Figure 1c). The last change detected during study reported here was a mutation in the gene fragment covering exon 15 in one patient from group 2 at position 187 409 (2532 in cDNA; NM_012464) changing A to G and resulting in a codon 629 for isoleucine being substituted to codon for valine (Figure 1d).
Discussion
Recently, numerous reports described mutations at the level of single genes, including NKX 2.5 and GATA4 in relation to heart developmental anomalies.38 Therefore, the lack of structural and numerical chromosomal abnormalities observed here is not surprising. Lack of mutations in exons 3 and 11 indicates that the structural parts of the protein encoded by these exons must be vital for the development. Indeed, the exon 3 encodes amino acids building central portion of the latency propeptide. Thus, we postulate that any change in this part of the peptide could alter the structure by the mean of its miss folding, which in turn could lead to serious problems in specific activation of the enzyme by putative furins.14 Retention of the latency peptide would be a reason for the lack of activity of TLL1.
The exon 11 encodes amino acids 421–460 being a part of the CUB1 domain. For proteinases homologous to TLL1, such as mTld and BMP-1, it has been shown that the CUB1 domain is critical for their secretion from the cells.39, 40 Lack of this domain or shuffling it into a different part of the recombinant protein led to the retention of such an aberrant product in the cytoplasm.39 Therefore, it is possible that mutations in the exon 11 would cause a complete lack or at least poor secretion of TLL1 in a human and to a natural abortion of the embryo because of the developmental abnormalities as has been reported for transgenic mice null for tll1.13
The mutation in codon at position 182 may affect conversion of the zymogen to the active enzyme. The methionine 182 is the first residue in the catalytic domain, which is conserved among all the astacins including maprins α and β (Figure 2a). It is also the first amino acid in the sequence with positions conserved for two cysteines in all known members of the astacin family (Figure 2a). Therefore, mutation of the hydrophobic residue such as Met182 to an aliphatic Leu residue, which occupies place in the first α helix of the catalytic domain41 might have a consequence in altering the structure of catalytic domain and affect the activity of TLL1 on its native substrate, chordin.
Figure 2.
Comparison of conserved amino acid sequence domains mutated in TLL1 with the corresponding domains in selected metzincins. (a) N-terminal end of the catalytic domain, (b) active centers of selected metalloproteases from astacin family and other selected metzincins. (c) N-terminal end of the CUB3 domain. Symbols: H, amino acid sequence of human polypeptides; M, mutated TLL1; dashes, identical residues as in the polypeptide aligned at the top of the alignment. Colors: yellow, conserved cysteines; pink, histidines coordinating zinc in the active center of the enzyme; red, amino acid sequence in extended catalytic center; green, CUB3 domains in different enzymes; blue, mutations detected in ASD patients.
Another mutation caused a change of residue at position 238, which is located within the catalytic domain, just before the zinc-binding signature (Figure 2b). The structure of this region is critical for substrate accommodation of the catalytic cleft. This cleft in all tolloids is deeper than the cleft in astacin.42, 43 A deeper cleft limits substrates that may fit into the active center of the enzyme to hydrolyze the specific peptide bound.42, 43 The conserved two residues preceding the zinc-binding signature in proteases classified in astacin family are always isoleucine or valine at first position and isoleucine, alanine, valine, glutamic acid or aspartic acid at second position. In other metzincins, such as MMPs, the two corresponding residues located immediately upstream of the zinc-binding signature are alanines. Substitution of the two Ile residues in astacin and mTld by valine and alanine in both TLLs might be related to change in substrate specificity of these metalloproteases. Except for MMPs the alanine is always present in the second position after the valine. In MMPs that have a broad spectrum of substrates, the two alanines, that are less bulky than valines, do not interfere with the accommodation of various substrates in the cleft of the active center. Thus, the change in the substrate specificity of TLL1 resulting from substitution of the bulkier valine by smaller alanine could allow competition for the binding of nonspecific substrates to the catalytic cleft. This, in turn, could result in lower enzymic efficacy of the mutated TLL1 in the regulation of heart septation during embryonic and fetal development, which would finally lead to the permanent ASD condition.
The CUB3 domain is the only domain present in all BMP-1 gene products as well as in both TLLs. A comparison of the sequence flanking the mutated residue in TLL1 shows almost complete conservation of the motif GX(I/L)T(S/T)PGWPKEYPNK (Figure 2c). This signature is also highly conserved among CUB2, 3, and 4 domains in mTld and TLLs with Ile preserved in all the CUBs except in CUB4 of TLL2. Such conservation indicates for functional importance of the peptide in the protein structure. The significance of the change in CUB3 of Val for Ile needs to be verified by obtaining such mutated enzyme and assaying its substrate specificity. Earlier reports indicated that intact structure consisting of CUB3 with both flanking EGF-like motifs are required in mTld for maintaining its activity on specific substrate.39, 40
The analysis of the TLL1 is, at present, time and labor consuming. The gene consists of 231 784 bp divided into 22 exons. The cDNA is 6654 bp long but the ORF consists only 3042 bp and it encodes a polypeptide of 1013 amino acids long. The 5′ upstream sequence of 647 bp is a large 5′UTR with four methionine codons of an unclear function. The STOP codon is followed by a 230 bp sequence reach in A and T stretches. Most of the coding sequence of this gene is enclosed in two large exons, E1 (816 bp) and E21/22 (3100 bp). The remaining sequence of 2738 bp is divided into 19 short exons of similar length. There is also a large intron 1, covering more than half of the gene sequence (116 123 bp). Its role has yet to be clarified.
Our observations also require, further epidemiological studies on larger and clinically more uniform group of ASD patients to determine the population frequency of mutations in TLL1 gene. The reason that we did not detect defects in all our ASD patients could be explained by the fact that some patients might have mutations in different genes known to be involved in heart development. In addition, familial cases of ASD with TLL1 mutations are yet to be detected in larger population studies that already have begun.
Finally, we have encountered serious problems in obtaining good quality DNA sequence data from the first half of exon 1 and from the large 3′ region of exon 21/22. Thus, it is still possible that in some patients there could be mutations resulting in frame shift and/or nonsense codon mutations. In addition, the promoter and other regulatory regions that we did not sequence in this study could contain mutations affecting gene expression levels. However, here, we have shown for the first time that mutations in TLL1 are linked to the same human syndrome, which was originally generated in transgenic animals by knocking out the gene.13 Thus, our results indicate that in some patients with ASD, the genetic causes of the syndrome are heterozygous mutations in TLL1.
Acknowledgments
The study was supported in part by the Grant no. 2P05D 059 27 from the Ministry of Sciences and Education, and by institutional funds NN-1-006/03, NN-4-042/04 and NN-1-014/06.
References
- Kahler RL, Braunwald E, Plauth WH, Jr, Morrow AG. Familial congenital heart disease: familial occurrence of atrial septal defect with A-V conduction abnormalities, supravalvular aortic and pulmonic stenosis, and ventricular septal defect. Am J Med. 1966;40:384–399. [Google Scholar]
- Boughman JA, Berg KA, Astemborski JA, et al. Familial risks of congenital heart defect assessed in a population based epidemiologic study. Am J Med Genet. 1987;26:839–849. doi: 10.1002/ajmg.1320260411. [DOI] [PubMed] [Google Scholar]
- Van Mierop LHS.Embryology of the atrioventricular canal region and pathogenesis of endocardial cushion defectsin Feldt RH, McGoon DC, Ongley PA, Rastelli GC, Titus JL, Van Mierop LHS (eds):Atrioventricular Canal Defects Philadelphia, PA: WB Saunders; 19761–12. [Google Scholar]
- Hoffman JI. Congenital heart disease: incidence and inheritance. Pediatr Clin North Am. 1990;37:25–43. doi: 10.1016/s0031-3955(16)36830-4. [DOI] [PubMed] [Google Scholar]
- Fyler DC.Atrial septal defect secundumin Fyler DC (ed):Nadas Pediatric Cardiology Boston, MA: Mosby Year Book Inc.1992513–524. [Google Scholar]
- Brand A, Keren A, Branski D, Abrahamov A, Stern S. Natural course of atrial septal aneurysm in children and the potential for spontaneous closure of associated septal defect. Am J Cardiol. 1989;64:996–1001. doi: 10.1016/0002-9149(89)90797-2. [DOI] [PubMed] [Google Scholar]
- Zetterqvist P, Turesson I, Johansson BW, Laurell S, Ohlsson NM. Dominant mode of inheritance in atrial septal defect. Clin Genet. 1971;2:78–86. doi: 10.1111/j.1399-0004.1971.tb00259.x. [DOI] [PubMed] [Google Scholar]
- Pease WE, Nordenberg A, Ladda RL. Familial atrial septal defect with prolonged atrioventricular conduction. Circulation. 1976;53:759–762. doi: 10.1161/01.cir.53.5.759. [DOI] [PubMed] [Google Scholar]
- Li Volti S, Distefano G, Garozzo R, Romeo MG, Sciacca P, Mollica F. Autosomal dominant atrial septal defect of ostium secundum type. Report of three families. Ann Genet. 1991;34:14–18. [PubMed] [Google Scholar]
- Benson DW, Basson CT, MacRae CA. New understandings in the genetics of congenital heart disease. Curr Opin Pediatr. 1996;8:505–511. doi: 10.1097/00008480-199610000-00015. [DOI] [PubMed] [Google Scholar]
- Johnson MC, Hing A, Wood MK, Watson MS. Chromosome abnormalities in congenital heart disease. Am J Med Genet. 1997;70:292–298. doi: 10.1002/(sici)1096-8628(19970613)70:3<292::aid-ajmg15>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Basson CT, Cowley GS, Solomon SD, et al. The clinical and genetic spectrum of the Holt-Oram syndrome (heart-hand syndrome) N Engl J Med. 1994;330:885–891. doi: 10.1056/NEJM199403313301302. [DOI] [PubMed] [Google Scholar]
- Clark TG, Conway SJ, Scott IC, et al. The mammalian Tolloid-like 1 gene, Tll, 1 is necessary for normal septation and positioning of the heart. Development. 1999;126:2631–2642. doi: 10.1242/dev.126.12.2631. [DOI] [PubMed] [Google Scholar]
- Takahara K, Brevard R, Hoffman GG, Suzuki N, Greenspan DS. Characterization of a novel gene product (mammalian tolloid- like) with high sequence similarity to mammalian tolloid/bone morphogenetic protein-l. Genomics. 1996;34:157–165. doi: 10.1006/geno.1996.0260. [DOI] [PubMed] [Google Scholar]
- Dumermuth E, Sterchi EE, Jiang W, et al. The astacin family of metalloendopeptidases. J Biol Chem. 1991;26:21381–21385. [PubMed] [Google Scholar]
- Mullins MC. Holy Tolloido: Tolloid cleaves SOG/Chordin to free DPP/BMPs. Trends Genet. 1998;14:127–129. doi: 10.1016/s0168-9525(98)01431-0. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, et al. Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-Like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- Ge G, Fernández CA, Moses MA, Greenspan DS. Bone morphogenetic protein 1 processes prolactin to a 17-kDa antiangiogenic factor. Proc Natl Acad Sci USA. 2007;104:10010–10015. doi: 10.1073/pnas.0704179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Wang EA, Wozney JM. Identification of transforming growth factor 13 family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci USA. 1990;87:9843–9847. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Labosky PA, Furuta Y, et al. Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by BMP1, a mammalian gene related to Drosophila tolloid. Development. 1996;122:3587–3595. doi: 10.1242/dev.122.11.3587. [DOI] [PubMed] [Google Scholar]
- Yan L, Pollock GH, Nagase H, Sarras MP., Jr A 25.7 × 103 Mr hydra metalloproteinase (HMP1), a member of the astacin family, localizes to the extracellular matrix of Hydra vulgaris in a head-specific manner and has a developmental function. Development. 1995;121:1591–1602. doi: 10.1242/dev.121.6.1591. [DOI] [PubMed] [Google Scholar]
- Childs SR, O'Connor MB. Two domains of the tolloid protein contribute to its unusual genetic interaction with decapentaplegic. Develop Biol. 1994;162:209–220. doi: 10.1006/dbio.1994.1079. [DOI] [PubMed] [Google Scholar]
- Mott JD, Thomas CL, Rosenbach MT, Takahara K, Greenspan DS, Banda MJ. Post-translational proteolytic processing of procollagen C-terminal proteinase enhancer releases a metalloproteinase inhibitor. J Biol Chem. 2000;275:1384–1390. doi: 10.1074/jbc.275.2.1384. [DOI] [PubMed] [Google Scholar]
- Goodman SA, Albano R, Wardle FC, Matthews G, Tannahill D, Dale L. BMP1-related metalloproteinases promote the development of ventral mesoderm in early Xenopus embryos. Develop Biol. 1998;195:144–157. doi: 10.1006/dbio.1997.8840. [DOI] [PubMed] [Google Scholar]
- Pappano WN, Scott IC, Clark TG, Eddy RL, Shows TB, Greenspan DS. Coding sequence and expression patterns of mouse chordin and mapping of the cognate mouse Chrd and human CHRD genes. Genomics. 1998;52:236–239. doi: 10.1006/geno.1998.5474. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of chordin by xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquès G, Musacchio M, Shimell MJ, Wünnenberg-Stapleton K, Cho KW, O'Connor MB. Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell. 1997;91:417–426. doi: 10.1016/s0092-8674(00)80425-0. [DOI] [PubMed] [Google Scholar]
- Panchenko MV, Stetler-Stevenson WG, Trubetskoy OV, Gacheru SN, Kagan HM. Metalloproteinase activity secreted by fibrogenic cells in the processing of prolysyl oxidase. Potential role of procollagen C-proteinase. J Biol Chem. 1996;271:7113–7119. doi: 10.1074/jbc.271.12.7113. [DOI] [PubMed] [Google Scholar]
- Takahara K, Kessler E, Biniaminov L, et al. Type I procollagen COOH-terminated proteinase enhancer protein: identification, primary structure, and chromosomal localization of the cognate human gene (PCOLCE) J Biol Chem. 1994;269:26280–26285. [PubMed] [Google Scholar]
- Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-l: the type 1 procollagen C-proteinase. Science. 1996;271:360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- Li SW, Sieron A, Fertala A, Hojima Y, Arnold WV, Prockop DJ. The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein-1. Proc Natl Acad Sci USA. 1996;93:5127–5130. doi: 10.1073/pnas.93.10.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J. Mouse mutants and cardiac development: new molecular insights into cardiogenesis. Circ Res. 1996;78:349–353. doi: 10.1161/01.res.78.3.349. [DOI] [PubMed] [Google Scholar]
- Olson EN, Srivastava D. Molecular pathways controlling heart development. Science. 1996;272:671–676. doi: 10.1126/science.272.5262.671. [DOI] [PubMed] [Google Scholar]
- Barch MJ, Lawrence HJ, Arsham MS.Peripheral blood culturein Barch MJ (ed):The ACT Cytogenetics Laboratory Manual, 2nd edition New York: Raven Press; 199117–30. [Google Scholar]
- Henegariu O, Heerema NA, Lowe Wright L, Bray-Ward P, Ward DC, Vance GH. Improvements in cytogenetic slide preparation: controlled chromosome spreading, chemical aging and gradual denaturing. Cytometry. 1991;43:101–109. [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Nat Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doevendans PA, van Bilsen M. Transcription factors and the cardiac gene programme. Int J Biochem Cell Biol. 1996;28:387–403. doi: 10.1016/1357-2725(95)00145-x. [DOI] [PubMed] [Google Scholar]
- Hartigan N, Garrigue-Antar L, Kadler KE. Bone morphogenetic protein (BMP-1): Identification of the minimal domain structure for procollagen C-proteinase activity. J Biol Chem. 2003;278:18045–18049. doi: 10.1074/jbc.M211448200. [DOI] [PubMed] [Google Scholar]
- Garrigue-Antar L, Francois V, Kalder KE. Deletion of epidermal growth factor-like domains converts mammalian tolloid into a chordinase and effective procollagen C-proteinase. J Biol Chem. 2004;279:49835–49841. doi: 10.1074/jbc.M408134200. [DOI] [PubMed] [Google Scholar]
- Gomis-Rüth FX, Stöcker W, Huber R, Zwilling R, Bode W. Refined 1.8 Å X-ray crystal strucutre of astacin, a zinc-endopeptidase from the crayfish Astacus astacus L. Structure determinantion, refinement, molecular structure and comparison with thermolysin. J Mol Biol. 1993;229:945–968. doi: 10.1006/jmbi.1993.1098. [DOI] [PubMed] [Google Scholar]
- Stöcker W, Ng M, Auld DS. Fluorescent oligopeptide substrates for kinetic characterization of the specificity of Astacus protease. Biochemistry. 1990;29:10418–10425. doi: 10.1021/bi00497a018. [DOI] [PubMed] [Google Scholar]
- Garrigue-Antar L, Barker C, Kadler KE. Identification of amino acid residues in bone morphogenetic protein-1 important for procollagen C-proteinase activity. J Biol Chem. 2001;276:26237–26242. doi: 10.1074/jbc.M010814200. [DOI] [PubMed] [Google Scholar]