Abstract
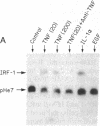
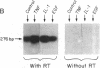
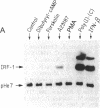
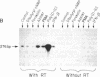
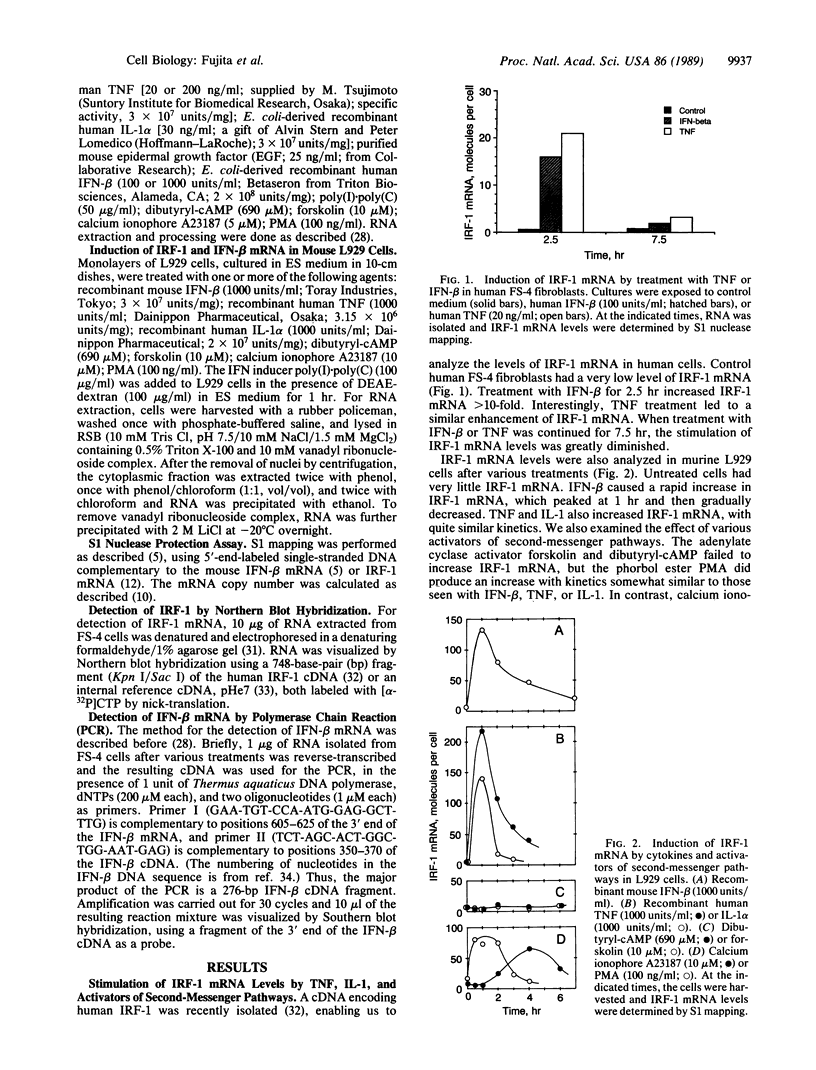
Nuclear protein IRF-1 (interferon regulatory factor 1) was earlier shown to bind to cis-acting regulatory elements present on interferon (IFN)-alpha/beta genes and some IFN-inducible genes. Here we show that in both human FS-4 and murine L929 cells, steady-state levels of IRF-1 mRNA were increased by treatment with tumor necrosis factor (TNF), interleukin 1 (IL-1), poly(I).poly(C), or IFN-beta. IRF-1 mRNA induction was also demonstrated in cells treated with calcium ionophore A23187 or with phorbol 12-myristate 13-acetate, but not with epidermal growth factor, dibutyryl-cAMP, or the adenylate cyclase activator forskolin. To determine whether stimulation of IRF-1 mRNA levels correlates with IFN-beta induction, we compared IRF-1 and IFN-beta mRNA levels in cells exposed to various stimuli. In L929 cells, treatment with poly(I).poly(C) under conditions that failed to induce significant levels of IFN-beta mRNA led to a very low induction of IRF-1 mRNA, but "priming" cells with IFN prior to the addition of poly(I).poly(C) greatly increased both IRF-1 and IFN-beta mRNAs. In FS-4 cells an increase in IFN-beta mRNA (examined by the polymerase chain reaction) was seen after treatment with TNF, IL-1, A23187, or poly(I).poly(C), but not with IFN-beta, epidermal growth factor, dibutyryl-cAMP, or forskolin. Thus, all treatments that increased steady-state levels of IFN-beta mRNA also enhanced IRF-1 mRNA levels. However, treatment with IFN-beta, which caused a marked stimulation in IRF-1 mRNA, failed to produce a detectable increase in IFN-beta mRNA. It appears that IRF-1 may be necessary but not sufficient for IFN-beta induction. The ability of TNF and IL-1 to increase both IRF-1 and IFN-beta mRNAs may be responsible for some similarities in the actions of TNF, IL-1, and the IFNs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babich A., Feldman L. T., Nevins J. R., Darnell J. E., Jr, Weinberger C. Effect of adenovirus on metabolism of specific host mRNAs: transport control and specific translational discrimination. Mol Cell Biol. 1983 Jul;3(7):1212–1221. doi: 10.1128/mcb.3.7.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresini M. H., Lempert M. J., Epstein L. B. Overlapping polypeptide induction in human fibroblasts in response to treatment with interferon-alpha, interferon-gamma, interleukin 1 alpha, interleukin 1 beta, and tumor necrosis factor. J Immunol. 1988 Jan 15;140(2):485–493. [PubMed] [Google Scholar]
- Clark L., Hay R. T. Sequence requirement for specific interaction of an enhancer binding protein (EBP1) with DNA. Nucleic Acids Res. 1989 Jan 25;17(2):499–516. doi: 10.1093/nar/17.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Lapierre L. A., Fiers W., Strominger J. L., Pober J. S. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986 Jan;83(2):446–450. doi: 10.1073/pnas.83.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Content J., DeClercq E., Volckaert G., Tavernier J., Devos R., Fiers W. Isolation and structure of a human fibroblast interferon gene. Nature. 1980 Jun 19;285(5766):542–547. doi: 10.1038/285542a0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Kimura Y., Miyamoto M., Barsoumian E. L., Taniguchi T. Induction of endogenous IFN-alpha and IFN-beta genes by a regulatory transcription factor, IRF-1. Nature. 1989 Jan 19;337(6204):270–272. doi: 10.1038/337270a0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Kohno S. Studies on interferon priming: cellular response to viral and nonviral inducers and requirement of protein synthesis. Virology. 1981 Jul 15;112(1):62–69. doi: 10.1016/0042-6822(81)90612-7. [DOI] [PubMed] [Google Scholar]
- Fujita T., Miyamoto M., Kimura Y., Hammer J., Taniguchi T. Involvement of a cis-element that binds an H2TF-1/NF kappa B like factor(s) in the virus-induced interferon-beta gene expression. Nucleic Acids Res. 1989 May 11;17(9):3335–3346. doi: 10.1093/nar/17.9.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Sakakibara J., Sudo Y., Miyamoto M., Kimura Y., Taniguchi T. Evidence for a nuclear factor(s), IRF-1, mediating induction and silencing properties to human IFN-beta gene regulatory elements. EMBO J. 1988 Nov;7(11):3397–3405. doi: 10.1002/j.1460-2075.1988.tb03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Hotta H., Yamanishi K., Taniguchi T. Interferon-beta gene regulation: tandemly repeated sequences of a synthetic 6 bp oligomer function as a virus-inducible enhancer. Cell. 1987 May 8;49(3):357–367. doi: 10.1016/0092-8674(87)90288-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Blanco M. A., Lengyel P., Morrison E., Brownlee C., Stiles C. D., Rutherford M., Hannigan G., Williams B. R. Regulation of 2',5'-oligoadenylate synthetase gene expression by interferons and platelet-derived growth factor. Mol Cell Biol. 1989 Mar;9(3):1060–1068. doi: 10.1128/mcb.9.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Maniatis T. Overlapping positive and negative regulatory domains of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1447–1451. doi: 10.1073/pnas.85.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G. E., Leung K., Folks T. M., Kunkel S., Nabel G. J. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature. 1989 May 4;339(6219):70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- Harada H., Fujita T., Miyamoto M., Kimura Y., Maruyama M., Furia A., Miyata T., Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989 Aug 25;58(4):729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Hirai M., Okamura N., Terano Y., Tsujimoto M., Nakazato H. Production and characterization of monoclonal antibodies to human tumor necrosis factor. J Immunol Methods. 1987 Jan 26;96(1):57–62. doi: 10.1016/0022-1759(87)90367-x. [DOI] [PubMed] [Google Scholar]
- Hug H., Costas M., Staeheli P., Aebi M., Weissmann C. Organization of the murine Mx gene and characterization of its interferon- and virus-inducible promoter. Mol Cell Biol. 1988 Aug;8(8):3065–3079. doi: 10.1128/mcb.8.8.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A. D., Maniatis T. Identification of an inducible factor that binds to a positive regulatory element of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 May;85(10):3309–3313. doi: 10.1073/pnas.85.10.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., May L. T., Tamm I., Vilcek J., Sehgal P. B. A cytokine network in human diploid fibroblasts: interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol. 1987 Jan;7(1):273–280. doi: 10.1128/mcb.7.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., Zhang Y. H., Lin J. X., Yamazaki S., Sehgal P. B., Vilcek J. Interleukin-1 can inhibit interferon-beta synthesis and its antiviral action: comparison with tumor necrosis factor. J Interferon Res. 1988 Aug;8(4):559–570. doi: 10.1089/jir.1988.8.559. [DOI] [PubMed] [Google Scholar]
- Kuhl D., de la Fuente J., Chaturvedi M., Parimoo S., Ryals J., Meyer F., Weissmann C. Reversible silencing of enhancers by sequences derived from the human IFN-alpha promoter. Cell. 1987 Sep 25;50(7):1057–1069. doi: 10.1016/0092-8674(87)90172-3. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Fan C. M., Maniatis T., Baltimore D. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell. 1989 Apr 21;57(2):287–294. doi: 10.1016/0092-8674(89)90966-5. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Reich N., Darnell J. E., Jr Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 1988 Apr;2(4):383–393. doi: 10.1101/gad.2.4.383. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Ballard D. W., Böhnlein E., Greene W. C. Tumor necrosis factor alpha induces proteins that bind specifically to kappa B-like enhancer elements and regulate interleukin 2 receptor alpha-chain gene expression in primary human T lymphocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2331–2335. doi: 10.1073/pnas.86.7.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M., Fujita T., Taniguchi T. Sequence of a cDNA coding for human IRF-1. Nucleic Acids Res. 1989 Apr 25;17(8):3292–3292. doi: 10.1093/nar/17.8.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestan J., Digel W., Mittnacht S., Hillen H., Blohm D., Möller A., Jacobsen H., Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. 1986 Oct 30-Nov 5Nature. 323(6091):816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Onozaki K., Urawa H., Tamatani T., Iwamura Y., Hashimoto T., Baba T., Suzuki H., Yamada M., Yamamoto S., Oppenheim J. J. Synergistic interactions of interleukin 1, interferon-beta, and tumor necrosis factor in terminally differentiating a mouse myeloid leukemic cell line (M1). Evidence that interferon-beta is an autocrine differentiating factor. J Immunol. 1988 Jan 1;140(1):112–119. [PubMed] [Google Scholar]
- Pfizenmaier K., Scheurich P., Schlüter C., Krönke M. Tumor necrosis factor enhances HLA-A,B,C and HLA-DR gene expression in human tumor cells. J Immunol. 1987 Feb 1;138(3):975–980. [PubMed] [Google Scholar]
- Reis L. F., Ho Lee T., Vilcek J. Tumor necrosis factor acts synergistically with autocrine interferon-beta and increases interferon-beta mRNA levels in human fibroblasts. J Biol Chem. 1989 Oct 5;264(28):16351–16354. [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Lunn R. M., Richardson N. K., Hellermann G. R., Smith L. J., Old L. J. Tumor necrosis factor and IFN induce a common set of proteins. J Immunol. 1988 Aug 15;141(4):1180–1184. [PubMed] [Google Scholar]
- Ryals J., Dierks P., Ragg H., Weissmann C. A 46-nucleotide promoter segment from an IFN-alpha gene renders an unrelated promoter inducible by virus. Cell. 1985 Jun;41(2):497–507. doi: 10.1016/s0092-8674(85)80023-4. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Regulation of cytokine gene expression. Annu Rev Immunol. 1988;6:439–464. doi: 10.1146/annurev.iy.06.040188.002255. [DOI] [PubMed] [Google Scholar]
- Tiwari R. K., Kusari J., Sen G. C. Functional equivalents of interferon-mediated signals needed for induction of an mRNA can be generated by double-stranded RNA and growth factors. EMBO J. 1987 Nov;6(11):3373–3378. doi: 10.1002/j.1460-2075.1987.tb02659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme J., Opdenakker G., Billiau A., De Somer P., De Wit L., Poupart P., Content J. Stimulation of fibroblast interferon production by a 22K protein from human leukocytes. J Gen Virol. 1985 Apr;66(Pt 4):693–700. doi: 10.1099/0022-1317-66-4-693. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Havell E. A. Stabilization of interferon messenger RNA activity by treatment of cells with metabolic inhibitors and lowering of the incubation temperature. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3909–3913. doi: 10.1073/pnas.70.12.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan K. V., Goodbourn S. Double-stranded RNA activates binding of NF-kappa B to an inducible element in the human beta-interferon promoter. EMBO J. 1989 Apr;8(4):1129–1138. doi: 10.1002/j.1460-2075.1989.tb03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathelet M. G., Clauss I. M., Nols C. B., Content J., Huez G. A. New inducers revealed by the promoter sequence analysis of two interferon-activated human genes. Eur J Biochem. 1987 Dec 1;169(2):313–321. doi: 10.1111/j.1432-1033.1987.tb13614.x. [DOI] [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]
- Zullo J. N., Cochran B. H., Huang A. S., Stiles C. D. Platelet-derived growth factor and double-stranded ribonucleic acids stimulate expression of the same genes in 3T3 cells. Cell. 1985 Dec;43(3 Pt 2):793–800. doi: 10.1016/0092-8674(85)90252-1. [DOI] [PubMed] [Google Scholar]