Abstract
Abdominal aortic aneurysm (AAA) is among a number of vascular disorders to be recently associated with a common allelic variant situated on chromosome 9p21. To further assess the significance of this region of the genome in AAA development, we genotyped the sequence variation tagged by rs10757278 in two geographically independent cohorts of patients and compared them to matched controls. We also assessed the impact of this variant on AAA growth rate in cohorts with a median surveillance period of 3.2 and 4.5 years. Using meta-analysis to combine the findings of both cohorts, we found a significant association between rs10757278-G and the presence of AAA (OR (95%CI) 1.38 (1.04–1.82) P=0.03), an effect size completely consistent with that originally reported. rs10757278 was not significantly associated with altered AAA growth rate in either cohort.
Keywords: aneurysm, genetic, variant, growth, chromosome 9p21
Introduction
Recently, a sequence variant on 9p21, tagged by rs10757278-G, was reported to be associated with the presence of abdominal aortic aneurysm (AAA; Odds ratio (OR) 1. 31 (1.22–1.42)) and intracranial aneurysm (OR 1.29 (1.16–1.43)) in a large multiple cohort study.1 Previously, genome-wide association studies had identified an association between this variant and myocardial infarction and coronary artery disease (CAD).2 Some pathological characteristics and risk factors for these disorders are shared, such as medial wall remodelling, atherosclerosis and hypertension, but there are distinct clinical and histological differences. In identifying this shared association with an as yet undetermined area of the genome, a search has begun to find a biological pathway/system that has a wide-reaching influence on arterial pathophysiology. Interestingly, an association of type 2 diabetes with a different variant in the same area of 9p21 has also been reported.3, 4, 5
We aimed to assess the association between AAA presence and growth with the 9p21 variant, using rs10757278 in two geographically distinct cohorts of subjects, while taking into account other risk factors for atherosclerotic disease.
Materials and methods
Patients
Patients were recruited from AAA screening programs carried out in Chichester, UK and Perth, Australia. AAA was defined by an infrarenal aortic diameter ≥3 cm, assessed using ultrasonography. Patients who had AAA were followed up with serial ultrasound scans to assess changes in maximum infrarenal aortic diameter. The Australian cohort was recruited from the Health in Men Study (HIMS) involving over 12 200 participants in a population-based trial of screening for AAAs in the state of Western Australia.6 Cases were age matched to controls without AAAs and normal aortic diameter (19–22 mm) from the same study. In the UK cohort, cases were matched for age and male gender (using a homogeneous strata within each group7) with controls from the Northwick Park Heart Study II (NPHSII), a prospective study of middle aged men recruited from nine UK general practices8 (for further details of recruitment, see Jones et al8).
Genotyping
Rs 10757278 was genotyped using TaqMan technology (Applied Biosciences (ABI), Warrington, UK) in the UK cohort, and the homogeneous MassEXTEND (hME; Sequenom) assay in the WA cohort.
Statistical analysis
Cases and controls were compared within cohorts using additive and dominant models. ORs were obtained from logistic regression models for the WA cohort and conditional logistic regression for the UK cohort. rs10757278 and CAD are associated and so are CAD and AAA. A subanalysis was performed in subjects who did not have a history of CAD to assess this potential confounding effect. Age, smoking history, hypertension, diabetes and hypercholesterolaemia (detailed in Table 1) were adjusted for with multiple logistic regression analysis. A random effects meta-analysis was performed to combine cohorts. AAA growth rate was estimated using the flexible hierarchical model described by Brady et al.9
Table 1. Demographics of the UK and WA cohorts.
| UK men | WA men | |||||
|---|---|---|---|---|---|---|
| Controls (N=985) | AAA cases excluding IHD (N=269) | AAA cases including IHD (N=386) | Controls (N=381) | AAA cases excluding IHD (N=205) | AAA cases including IHD (N=355) | |
| Age, mean (SD) | 72.0 (2.7) | 70.3 (4.0) | 70.4 (3.9) | 73.4 (4.5) | 73.4 (4.5) | 73.4 (4.4) |
| Hypertension % | 53.8 | 68.2** | 62.2** | 44.9 | 49.8* | 55.1** |
| Diabetes % | 7.7 | 7.9 | 8.9 | 7.7 | 10.9 | 12.0 |
| Hypercholesterolaemia % | 20.7 | 56.1** | 51.2** | 27.7 | 32.2 | 44.0* |
| Smoking history % | ||||||
| Ever % | 66.1 | 91.2** | 90** | 65.5 | 82.9** | 87.7** |
| History of CAD, % (N) | 27.3 | 0 | 30.5 | 19.0 | 0 | 39.3** |
| Median initial AAA diameter (IQR) | 35 (30–43) | 35 (30–43) | 34 (31–38) | 34 (31–39) | ||
| Median final AAA diameter (IQR) | 51 (37–57) | 51 (38–57) | 41 (35–47) | 41 (35–47) | ||
| Median surveillance period (IQR) | 3.0 (1.2–6.4) | 3.2 (1.4–6.9) | 4 (2–5.5) | 4.5 (2–5.5) | ||
| Median growth rate (95% reference range) | 2.1 (−1.0 to 5.3) | 2.2 (−1.5 to 5.9) | 1.25 (−1.5 to 4.0) | 1.27 (−1.8–4.3) | ||
*P<0.01 and **P<0.001 when compared with control group.
Results
Case–control series from the United Kingdom and Western Australia were examined in this study, resulting in comparison of a total of 741 cases and 1366 controls (Table 1). rs10757278G allele variation was not significantly associated with AAA in either case–control series when analysed separately (in the United Kingdom, OR=1.21 (0.81–1.82))and in the Western Australia, OR=1.40 (0.96–2.05; Table 2), but in the combined cohorts (excluding AAA subjects with known CAD), G-allele carriers had higher risk of AAA disease, than AA subjects (OR 1.38 (95% CI; 1.04–1.82) P=0.03, data adjusted for potential CHD confounders). In both cohorts, excluding those with CAD, rs10757278 was not associated with altered AAA expansion (in the United Kingdom, 0.21 mm (−0.33 to 0.76) P=0.45, in the Western Australia, 0.30 mm (−0.11 to 0.72) P=0.15). Combining expansion data for both the cohorts did not reach statistical significance (P=0.11).
Table 2. Genotype distributions, allele frequencies and odds ratios (ORs) of rs10757278A>G case–control comparisons in the two cohorts.
| Genotype | Controls excluding IHD (UK age/sex stratified) | AAA cases excluding IHD | OR (95% CI)a | Controls including IHD (UK age/sex stratified) | AAA Cases including IHD | OR (95% CI)a | |
|---|---|---|---|---|---|---|---|
| UK | AA | 200 (28.3) | 64 (24.3) | 1.00 | 256 (26.0) | 96 (25.7) | 1.00 |
| AG | 352 (49.9) | 139 (52.9) | 1.24 (0.86–1.79) | 510 (51.8) | 190 (50.8) | 1.20 (0.78–1.85) | |
| GG | 154 (21.8) | 60 (22.8) | 1.30 (0.83–2.01) | 219 (22.2) | 88 (23.5) | 1.24 (0.75–2.04) | |
| P=0.43 | P=0.65 | ||||||
| G allele freq. (95% CI) | 0.467 (0.441–0.494) | 0.492 (0.449–0.536) | GG+GA vs AA, 1.26 (0.88–1.79) | 0.481 (0.459–0.504) | 0.489 (0.453–0.526) | GG+GA vs AA, 1.21 (0.81–1.82) | |
| P=0.20 | P=0.36 | ||||||
| WA | AA | 71 (25.6) | 36 (18.3) | 1.00 | 100 (25.6) | 75 (21.6) | 1.00 |
| AG | 135 (48.7) | 110 (55.8) | 1.67 (1.04–2.70) | 186 (47.6) | 189 (54.3) | 1.49 (1.00–2.22) | |
| GG | 71 (25.6) | 51 (25.9) | 1.45 (0.84–2.49) | 105 (26.9) | 84 (24.1) | 1.23 (0.78–1.94) | |
| P=0.10 | P=0.08 | ||||||
| G allele freq. (95% CI) | 0.500 (0.458 –0.542) | 0.538 (0.487–0.588) | AG+GG vs AA, 1.59 (1.01–2.52) | 0.506 (0.477–0.542) | 0.513 (0.475–0.551) | AG+GG vs AA, 1.40 (0.96–2.05) | |
| P=0.05 | P=0.08 |
Adjusted for covariates (Table 1).
Discussion
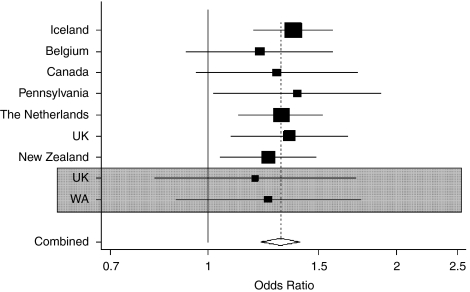
Individually the UK and not the WA case vs control cohort were sufficiently powered to assess an association between AAA and rs10757278 (in the United Kingdom, 88.7% and in WA, 73.1% power to detect a difference at 5% significance, based on the findings of Helgadottir et al1). However, the reported association between rs10757278-G and AAA,1was only confirmed statistically (P>0.03) in the combined group with an overall effect of 1.38 (1.04–1.82), demonstrating the importance of combining cohorts to improve power in candidate gene analysis. When patients with CAD were excluded from the analysis as a potential confounder, the sizes of the effects were essentially unchanged. The size of the association found in our study is consistent with that of Helgadottir et al1(Figure 1), suggesting that the original report did not overestimate the true effect, as has been described for other associations.10 Combining the published data and ours (3558 cases and 18108 controls), results in an overall is OR=1.31 (1.22–1.41, P<0.0001).
Figure 1.
Meta-analysis combining odds ratios for rs10757278-G and AAA association, with current data (highlighted in grey) and previously published data.1 Combined odds ratio is 1.31 (1.22–1.41) P<0.0001. Test for heterogeneity between studies P=0.99 (3558 cases and 18108 controls).
We also assessed the relationship between the 9p21 variant and AAA growth rate using the previously employed multilevel model.9 Helgadottir et al1 reported a borderline significant association between the rs10757278-G allele and slower AAA growth in the UK small aneurysm trial cohort (AAA growth GG compared with AG −0.46 mm/year, P=0.05), and suggested that the sequence variation predisposed to atherosclerosis (important in AAA) formation but subsequently slowed AAA growth. This theory was supported by previous data from the UK small aneurysm trial, which was associated reduced ankle pressure with slower AAA progression.9 In contrast, in both of our cohorts (combined power >80% to detect 0.46 mm/year decrease in AAA growth rate, at the 5% level), there was no significant association between AAA growth and rs10757278-G allele. The smaller initial mean aortic diameters and longer surveillance periods in our patients than patients in the UK small aneurysm trial may explain the difference between these observations; clearly larger studies will be needed to confirm any genotype effect on AAA growth, which at best appears to be modest. This can only be achieved through the collaboration of AAA surveillance data.
Our confirmation of the association between AAA and the 9p21 variant has added to the urgency to understand this highly significant region of the genome for both occlusive and degenerative vascular disease. It might be expected that different genes would play a role in the progression of disease, which in any case appears to be more complex to assess.
Acknowledgments
The UK cohort received support from the British Heart Foundation (FS/04/012:RG2005/014) and the Scott Research Unit, Chichester. The WA study was supported by NHMRC Project Grant 303232 and National Institutes of Health Grant 1R01HL080010-01. Special thanks to all men and staff who participated in the Chichester AAA screening program, WA AAA Program and the Health in Men Study. PN and JG are supported by NHMRC Practitioner Fellowships 458505 and 431503. We thank Mrs Vicky Phillips for her highly skilled technical assistance and patient recruitment. Hilary Ashton and Stephanie Druce, Scott Research Unit, Chichester, for their support in patient recruitment and data collection. Ms Jade Hampel for genotyping assistance of the Australian samples and staff at the cardiovascular genetics department, UCL for their technical support in genotyping the UK cohort.
References
- Helgadottir A, Thorleifsson G, Magnusson KP, et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- McPherson R, Pertsemlidis A, Kavaslar N, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329:1259. doi: 10.1136/bmj.38272.478438.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GT, Thompson AR, van Bockxmeer FM, et al. Angiotensin II type 1 receptor 1166C polymorphism is associated with abdominal aortic aneurysm in three independent cohorts. Arterioscler Thromb Vasc Biol. 2008;28:764–770. doi: 10.1161/ATVBAHA.107.155564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GJ, Bauer KA, Barzegar S, et al. Increased activation of the haemostatic system in men at high risk of fatal coronary heart disease. Thromb Haemost. 1996;75:767–771. [PubMed] [Google Scholar]
- Brady AR, Thompson SG, Fowkes FG, et al. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- Zollner S, Pritchard JK. Overcoming the winner's curse: estimating penetrance parameters from case-control data. Am J Hum Genet. 2007;80:605–615. doi: 10.1086/512821. [DOI] [PMC free article] [PubMed] [Google Scholar]