Abstract
Treacher Collins syndrome (TCS) is a rare congenital disorder of craniofacial development that arises as the result of mutations in the TCOF1 gene, which encodes a nucleolar phosphoprotein known as Treacle. Individuals diagnosed with TCS frequently undergo multiple reconstructive surgeries, which are rarely fully corrective. Identifying potential avenues for rescue and/or repair of TCS depends on a profound appreciation of the etiology and pathogenesis of the syndrome. Recent research using animal models has not only determined the cellular basis of TCS but also, more importantly, unveiled a successful avenue for therapeutic intervention and prevention of the craniofacial anomalies observed in TCS.
Keywords: Treacher Collins syndrome, Tcof1/Treacle, neural crest cells, craniofacial, ribosome biogenesis, p53
In brief
TCS is a severe disorder of craniofacial development
TCS occurs with an incidence of 1:50 000 live births
TCS exhibits autosomal dominant inheritance with variable penetrance
TCS arises solely as the result of mutations in TCOF1; 60% of cases being spontaneous and 40% familial
There is no genotype/phenotype correlation
General cranioskeletal hypoplasia occurs due to generation of insufficient neural crest cells
Insufficient neural crest cell number is a consequence of neuroepithelial progenitor cell death
Tcof1/Treacle is an important spatiotemporal regulator of ribosome biogenesis
Haploinsufficiency of Tcof1/Treacle results in deficient ribosome biogenesis, which is incapable of meeting the proliferative needs of the neuroepithelium.
Deficient ribosome biogenesis leads to nucleolar stress activation and stabilization of p53, which causes the high degree of neuroepithelial apoptosis and consequent loss of neural crest cells
In an experimental animal-based strategy, genetic and/or chemical inhibition of p53-dependent apoptosis can restore the normal complement of neural crest cells and prevent the development of TCS craniofacial anomalies
Introduction
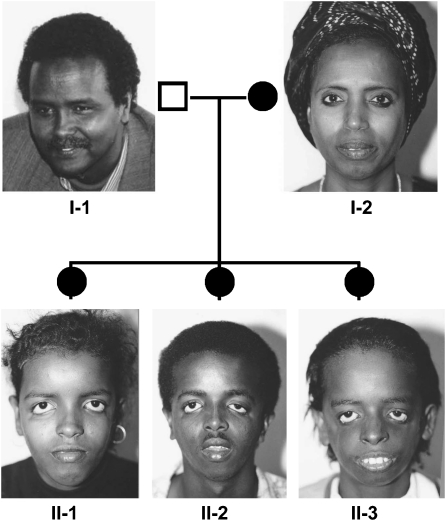
Treacher Collins syndrome (TCS, OMIM number 154500) is an autosomal dominant disorder of craniofacial morphogenesis (Figure 1). Also known as mandibulofacial dysostosis and Franceschetti-Zwahlen-Klein syndrome, TCS occurs with an estimated incidence of 1/50 000 live births.1, 2 Genetic, physical and transcript mapping techniques previously identified the gene mutated in TCS, designated TCOF1, which was found to encode a low complexity, serine/alanine-rich, nucleolar phosphoprotein termed Treacle (Supplementary Figure 1).3 Subsequently, more than 130 distinct mutations have been identified. The mutations that have been described to date arise throughout the gene and are predominantly family specific; and those documented include insertion, splicing and non-sense mutations. Deletions which range in size from 1 to 40 nucleotides are by far the most common.4 Although the causative mutations in a subset of patients have not been identified, TCS is thought to be genetically homogeneous because all the multigenerational families analyzed to date exhibit linkage to the human chromosome 5q32 locus. Intriguingly, however, 60% of the cases do not appear to have a previous family history and are thought to arise as the result of a de novo mutation.5 Penetrance of the genetic mutations underlying TCS is high yet extreme inter- and intra-familial variation in the severity of the phenotype (Figure 2) is a striking feature of the condition.6, 7 Individuals can be so mildly affected that it can be difficult to establish an unequivocal diagnosis and it is not uncommon for mildly affected TCS patients to be diagnosed retrospectively after the birth of a more severely affected child; this observation implies that the frequency of non-penetrance is under-reported. In contrast, at the other end of the clinical spectrum, severe cases of TCS have resulted in perinatal death.8 Collectively, the variable severity indicates that genetic background, environmental factors and stochastic events contribute to the clinical variation observed in TCS patients.9
Figure 1.
Diagnostic algorithm summarizing the morphological and genetic identification of individuals with Treacher Collins syndrome and their subsequent management. By permission of Oxford University Press, Inc.
Figure 2.
Clinical photographs and partial pedigree of a Somalian family. Individual I-2, who has an extensive family history of TCS, exhibits no apparent clinical features of mandibulofacial dysostosis. In contrast, all three children exhibit severe craniofacial anomalies consistent with TCS and furthermore share the same mutation (c.2259delA) as I-2. Adapted from chapter ‘Treacher Collins syndrome' by Dixon, Trainor and Dixon from ‘In born Errors of Development' edited by Epstein, Erickson and Wynshaw-Boris (2008). By permission of Oxford University Press, 2008.4
Recently, substantial advances have been made in our understanding of the cellular and biochemical pathogenesis of TCS. In particular, this review discusses the critical role of Tcof1/Treacle in ribosome biogenesis, which underpins neuroepithelial survival and neural crest cell proliferation that is central to normal craniofacial development. More importantly, a clear link has now been established between ribosome biogenesis, nucleolar stress activation of p53 and neuroepithelial apoptosis, the inhibition of which provides an exciting avenue for the therapeutic prevention of TCS.
Clinical overview
Treacher Collins syndrome is a severe congenital disorder of craniofacial development characterized by numerous developmental anomalies that are restricted to the head and neck (Figure 2). Hypoplasia of the facial bones, particularly the mandible (78% of cases) and zygomatic complex (81%), is an extremely common feature of TCS. Hypoplasia of the facial bones may result in dental malocclusion, with anterior open bite a common finding. The teeth may be widely spaced, malpositioned or reduced in number. In a large proportion of cases, the palate is high, arched and occasionally cleft (28%) and in severe cases, the zygomatic arches may be completely absent (Poswillo, 1975). Ophthalmic abnormalities include downward slanting of the palpebral fissures (89%) with notching of the lower eyelids (69%) and a paucity of lid lashes medial to the defect (69%) (Figure 2). Other clinical features of TCS include alterations in the shape, size and position of the external ears, which are frequently associated with atresia of the external auditory canals and anomalies of the middle ear ossicles. Radiographic analysis of the middle ears of TCS patients has revealed irregular or absent auditory ossicles with fusion between rudiments of the malleus and incus, partial absence of the stapes and oval window, or even complete absence of the middle ear and epitympanic space.10 Consequently, bilateral conductive hearing loss is common in TCS patients, whereas mixed or sensorineural hearing loss is rare.11
Diagnostic approaches
Before the identification of the TCOF1 gene, diagnosis of TCS was possible only by linked polymorphic markers and clinical evaluation (Dixon et al, 1994). However, in any clinical evaluation, the entire facial appearance needs to be considered when trying to arrive at a TCS diagnosis, particularly in mildly affected patients as there is a high degree of both inter- and intra-familial variability.6, 7 A number of attempts have been made to classify the condition based on the severity of affected features; however, these classifications are arbitrary. In most TCS patients, a spectrum of affected features is observed; in fact, rarely is any single abnormality alone sufficient to lead to a diagnosis of TCS.
The extreme variability in the degree to which individuals can be affected, together with the high rate of de novo mutations makes the provision of genetic counseling extremely complicated, particularly where the diagnosis of either of an affected child's parents is equivocal. In such cases, it is extremely important to ensure that neither parent manifests even minimal features of TCS. In this regard, the use of craniofacial radiographs, particularly the occipitomental view that facilitates visualization of the zygomatic complex, has proved extremely useful in detecting zygomatic hypoplasia.7, 12
A number of conditions exhibit phenotypic overlap with TCS, including Nager and Miller syndrome. Although it is usually straightforward to differentiate these conditions from TCS on the basis of the facial gestalt, caution should be exercised where individuals are only mildly affected so that the minimal diagnostic criteria that constitute TCS are not overlooked. Nager syndrome has similar facial features to TCS, particularly in the region of the eyes that are downslanting with a deficiency of eyelashes.13 However, the mandible is usually more hypoplastic than in TCS. The lower lid colobomas are rare, but preaxial limb abnormalities are a consistent feature of Nager syndrome, unlike TCS. Thumbs may be hypoplastic, aplastic, or duplicated and the radius and ulna may be fused. Cases of Nager syndrome are generally sporadic, although affected siblings have been reported in rare cases.13
Miller syndrome also has features in common with TCS, with the additional diagnostic feature of ectropion or out-turning of the lower lids.14 The cleft lip, with or without cleft palate, is more common than in TCS. The limb anomalies are post-axial, most commonly with absence or incomplete development of the fifth digital ray of all four limbs. In addition, there may be shortening of the radius and ulna. Some patients may exhibit congenital heart defects. The inheritance of Miller syndrome is somewhat unclear, as both autosomal dominant with variable expression15 and autosomal recessive forms16 have been reported.
The identification of the TCS locus, facilitated pre- and post-natal molecular diagnoses. Molecular analyses have revealed that TCOF1 consists of a 4233 bp open reading frame spanning 26 exons in which over 130 largely family-specific mutations have been documented, the bulk of which result in the introduction of a premature termination codon into Treacle. The wide spectrum of mutations observed in TCS complicates the provision of prenatal molecular diagnosis because it is necessary to identify the mutation in each family before undertaking diagnostic predictions. In any event, although molecular analysis has proven to be extremely valuable in prenatal diagnosis, it is not possible to predict how severely affected a fetus might be using this approach alone; consequently, ultrasonography is an invaluable aid to prenatal diagnosis, as this technique may provide information about the severity of affected pregnancies and can be used to evaluate fetal progression.8
Management
The care of individuals affected by TCS requires a multidisciplinary approach and may involve intervention from a number of health-care professionals both pre-and post-operatively17 (Figure 1). Of primary concern are breathing problems, which may arise at birth as a consequence of micrognathia and tongue obstruction of the hypopharynx. Emergency surgery in the form of a tracheostomy may be essential to maintain an adequate airway. Subsequent management of the hard and soft tissues typically requires multiple surgeries, and initially, depending on severity, eyelid coloboma and palatal clefting are corrected in the earliest years of life. This is followed by orbital reconstruction at about 5–7 years of age when most of the eye socket growth is complete and, if necessary, mandibular distraction or maxillo-mandibular osteotomies may be performed around the same time. In addition to multiple surgeries aimed at correcting under-developed or abnormal facial structures, patients may exhibit a range of symptoms, such as hearing loss and speech problems, which can have a significant impact upon learning ability, self-esteem and social interaction. Hence, it is critical to have a child's hearing tested at an early age, particularly before the first birthday, as this is critical for speech development. Ensuing problems can be lessened by implantation of appropriate bone-anchored conductive hearing devices. Reconstruction of the external and inner ear usually can be attempted at around the age of 6 years, however, depending on severity, titanium screw mounted prosthetic ears can achieve results esthetically superior to surgery. Although the results can be variable, excellent outcomes are achievable through a comprehensive, well coordinated and integrated treatment plan incorporating craniofacial surgeons, orthodontists, ophthalmologists, otolaryngologists and speech pathologists (Figure 1).
Molecular and genetic basis of the disease
Several hypotheses have been proposed to explain the cellular basis of TCS. These early theories included abnormal patterns of neural crest cell migration,18 abnormal domains of cell death,19, 20 improper cellular differentiation during development21 or an abnormality of the extracellular matrix;22 however, there was little experimental evidence to support any of these hypotheses. The integration of molecular biology, cell biology, mouse genetics and experimental embryology has recently provided novel insights into the molecular pathogenesis of TCS.
Cellular basis of TCS
Neural crest cells are a migratory cell population derived from the neuroepithelium during early embryogenesis that ultimately give rise to the majority of the cartilage, bone, and connective tissue of the head and face. Thus, most craniofacial anomalies, such as those associated with TCS, are thought to arise due to defects in neural crest cell formation, proliferation, migration and/or differentiation. Interestingly, the Tcof1 gene is spatiotemporally expressed in the neuroepithelium and in the neural crest-derived facial mesenchyme during early mouse embryogenesis, implying it plays a role in the development of these tissues.23
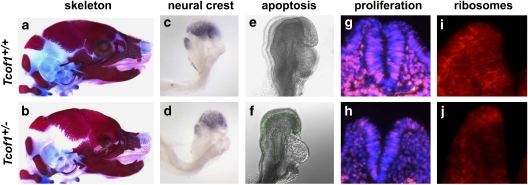
Contrary to previous hypotheses,18 cell lineage tracing performed in mouse models of TCS exhibiting severe craniofacial hypoplasia and dysplasia (Figure 3a and b) revealed no migratory nor path finding defects in cranial neural crest cell migration.23 This observation indicated that Tcof1 does not play a role in neural crest cell migration and, furthermore, that aberrant neural crest cell migration is not the underlying cause of TCS. However, despite the absence of a migration defect, 25% fewer migrating neural crest cells were reproducibly observed in TCS embryos compared with their wild-type littermates (Figure 3c and d), which accounts for the general cranioskeletal hypoplasia observed in mice and humans affected by TCS.23 This deficiency in neural crest cell number arises due to extensive neuroepithelial apoptosis (Figure 3e and f), which compromises the formation of neural crest cells. As a corollary to the elevated levels of apoptosis observed specifically in the neuroepithelium of TCS embryos, rates of proliferation were also examined. This surprisingly revealed that not only was proliferation reduced in the neuroepithelium but it was also compromised in the migrating neural crest cells (Figure 3g and h). Thus, Tcof1/Treacle is critically required for neuroepithelial survival and neural crest cell proliferation.23
Figure 3.
Developmental basis of TCS craniofacial anomalies. Comparative skeletal stains of E18.5 wild-type embryos (a) revealed severe frontonasal hypoplasia in Tcof1+/− littermates (b). Sox10 in situ hybridization of migrating neural crest cells in an E8.5 wild-type embryo (c) uncovered a severe reduction in the neural crest cell population in Tcof1+/− embryos (d). Staining for apoptosis (TUNEL:green) showed low endogenous levels of cell death in E8.5 wild-type embryos (e) and massively elevated levels in Tcof1+/− littermates (f). BrdU incorporation (red) into DAPI-stained nuclei (blue) of E9.0 embryos (g) highlighted a concomitant decrease in cell proliferation in Tcof1+/− embryos (h). Immunostaining for the 28S ribosomal protein (red) in E8.5 embryos (i) uncovered deficient ribosome biogenesis in Tcof1+/− littermates (j). Adapted from Dixon et al (2006).23
Genetic and biochemical basis of TCS
TCOF1 encodes the nucleolar phosphoprotein Treacle, which is a very simple protein containing few motifs of known function (Supplementary Figure 1). Treacle consists of three distinct domains, unique amino and carboxy termini and a characteristic central repeat domain.24, 25 The carboxy terminus of Treacle contains multiple nuclear localization signals, which have been shown to drive nuclear import of the protein.26, 27 Biochemical assays have determined that Treacle is highly phosphorylated and contains a series of repeat units that have been identified within individual exons containing a number of potential sites for casein kinase II phosphorylation and protein kinase C phosphorylation, suggesting that phosphorylation is important for the correct function of the protein.
Treacle is structurally most similar to Nopp140, which mediates pre-ribosomal ribonucleoprotein (pre-rRNP) export from the nucleus and ribosomal protein import from the cytoplasm. Immunofluorescence studies using anti-Treacle antibodies have localized Treacle to the dense fibrillar component of the nucleus28 and furthermore revealed that Treacle colocalizes with upstream binding factor and RNA polymerase1 in nucleolar organizing regions where it functions in ribosomal DNA gene transcription.29 More recently, Treacle has been identified as a component of human Nop65p-associated pre-ribosomal ribonucleoprotein (pre-rRNP) complexes30 that 2′-O-methylates pre-ribosomal RNA during the early stages of pre-RNA processing in the nucleolus.29 These data imply that Treacle is contained within an RNP complex in the nucleolus and may be involved in governing specific stages of the ribosome biogenesis process.
Indeed, consistent with its nucleolar localization, Treacle has been shown to play key roles in ribosome maturation and in so doing regulate neuroepithelial survival and neural crest cell proliferation.23, 29 Haploinsufficiency of Tcof1 leads to deficient ribosome biogenesis as measured by the production of the mature 28S subunit in neuroepithelial cells and neural crest cells (Figure 3i and j). This deficiency, which is insufficient to meet the demands of these highly proliferative cell populations, results in nucleolar stress activation of p53.31 p53 stabilization in turn transcriptionally activates numerous proapoptotic effector genes, such as Ccng1, Trp53inp1, Noxa, Perp and Wig1, within the neuroepithelium, which collectively are responsible for the high levels of tissue-specific death observed in the pathogenesis of TCS.31
Prevention of TCS craniofacial anomalies
The direct correlation between nuclear stabilization of p53 protein, activation of p53-dependent gene transcription and the induction of neuroepithelial apoptosis satisfactorily explains the observed deficiency in migrating neural crest cells in TCS.31 More importantly, however, this raised the intriguing possibility of preventing the onset and pathogenesis of TCS in vivo by blocking p53 function. Indeed, genetic inhibition of p53 as well as chemical inhibition of p53 by treating pregnant dams in utero with pifithrin-a successfully blocked Ccgn1 activity and the early phase of neuroepithelial apoptosis in TCS individuals during embryogenesis (Figure 4a–c). Moreover, this approach restored the migrating neural crest cell population (Figure 4d–f), thereby preventing cranioskeletal hypoplasia (Figure 4g–i), which consequently resulted in normal postnatal craniofacial development (Figure 4j and k).31
Figure 4.
Prevention of TCS through diminishment of p53 function. Activated capase3 immunostaining revealed low levels of cell death in E9.0 Tcof1+/+ (a) embryos, elevated levels in Tcof1+/− (b) and partially reduced levels in Tcof1+/−; p53+/− (c) littermates. Sox10 in situ hybridization labeled migrating neural crest cells in E9.5 Tcof1+/+ (d) embryos highlighting the reduction in Tcof1+/− (e) and restoration in Tcof1+/−; p53+/− (f) littermates. Bone (red) and cartilage (blue) staining showed normal cranioskeletal patterning in E18.5 Tcof1+/+ (g) embryos, severe frontonasal hypoplasia and dysplasia in Tcof1+/− (h) and complete rescue in Tcof1+/−; p53+/− (f) littermates. Bright field photograph of a 3-month-old Tcof1+/+ mouse (j) and a rescued post-natal viable and fertile Tcof1+/−; p53+/− (k) littermate. Adapted from Jones et al (2008).31
A major surprise arising from these experiments was that the pharmacological and genetic inhibition of p53 that was so successful in inhibiting neuroepithelial apoptosis occurred without altering or restoring ribosome biogenesis.31 Therefore, of central importance to a more profound understanding of TCS and its management in the future is the identification and characterization of novel functions of TCOF1/Treacle, which are not connected to the process of ribosome biogenesis. In this regard, it is interesting that the Treacle protein contains a consensus nuclear export signal sequence between amino acid positions 40–49.32 Furthermore, Treacle has also been shown to possess a LisH (Lis1 – homolgous motif) in its N-terminal region33 (Supplementary Figure 1). LisH motif-containing proteins are associated with microtubule binding and have been localized at centrosomes implicating them in microtubule dynamics, chromosome segregation and cell migration.34, 35 However, to date, no functional data have demonstrated that Treacle protein is exported from the nucleus. Nonetheless, the identification of individuals with mutations solely in the LisH domain of Treacle exhibiting unequivocal features of TCS implies that Treacle may shuttle between the nucleolus and cytoplasm. Disruptions to this shuttling process or interference with as yet unknown cytoplasmic functions for TCOF1/Treacle are thus potentially critical factors in the pathogenesis of characteristic TCS craniofacial abnormalities.
Conclusion
The major challenges facing the TCS clinical and research community in terms of improving the prognosis of affected or at risk individuals reside in three key areas; detection, repair and prevention. However, a key element limiting the strategies available is the extremely low incidence (1:50 000) of mutations in TCOF1, which is further compounded by the fact that 60% of the mutations arise spontaneously. This makes routine genetic screening for TCOF1 mutations during early gestation economically unviable except in families with a known history of TCS. Nevertheless, even in families with a history of TCS, the outcome of a positive identification during gestation is fraught with uncertainty due to the absence of a genotype/phenotype correlation. Consequently, the majority of individuals with craniofacial anomalies are detected during mid-to-late gestation through ultrasound screening, but confirming the anomalies are specifically TCS still requires genetic screening.
Technological advances in sonography have facilitated accurate in utero diagnoses of craniofacial malformations as early as 24 weeks of gestation with respect to cleft lip/palate, micrognathia, holoprosencephaly and craniosynostosis.8, 36, 37 Importantly, the technology available is sufficiently sophisticated to distinguish cleft lip/palate from cleft lip and there is a good correlation between prenatal and postnatal diagnoses. As ultrasound technology continues to improve so will the accuracy of prenatal detection of craniofacial abnormalities. Advances in magnetic resonance imaging, which in many instances is already being used in combination with ultrasonography, will also further advance the early prenatal detection of craniofacial anomalies. However, the onset of TCS abnormalities occurs very early during human embryonic development, typically within the first 4–8 weeks and phenotypic diagnosis at this stage even with the most sophisticated ultrasonography available today is impossible no matter the skill level of the professional ultrasonographer. Therefore, gestational diagnosis of TCS leaves multiple surgeries as the only available treatment option, and most craniofacial treatment centers have established timetables for postnatal surgical correction of palatal clefting and mandibular hypoplasia, which are based on severity and necessity balanced with the growth and development of the affected individual. However, despite the multiple rounds of surgery that a TCS patient typically endures, rarely are they fully corrective.
One possibility for improving the surgical outcome might be the incorporation of stem cells in therapeutic treatment of craniofacial abnormalities. There is enormous potential in the application of stem cells in engineering tissues, such as bone and cartilage, that constitute the head and facial tissues so severely disrupted in TCS. Taking this one step further, one could even envision the therapeutic application of stem cells in utero to treat some of the debilitating malformations associated with TCS. Fetal surgery became technically feasible during the 1980s38 and intrauterine repair of cleft palate, for example, is possible both in theory and practice.39, 40 However, at present, there is no universal recommendation for the in utero correction of prenatally diagnosed craniofacial anomalies and the decision to pursue fetal surgical intervention carries with it the potential for dual mortality (mother and fetus). Thus, fetal surgery at present remains experimental and controversial.
The numerous limitations in detection and repair of TCS leave prevention as the most promising alternative therapeutic avenue. However, methods of prevention are also not without problems. It is clear in animal models that chemical and genetic inhibition of p53 function can repress the wave of neuroepithelial apoptosis associated with TCS and in doing so prevents the pathogenesis of craniofacial anomalies. However, p53 performs many critically important cellular functions and suppressing p53 function completely is a very risky approach given that loss-of-function mutations in p53 are the most common mutations associated with cancer and tumorigenesis.41, 42, 43 Therefore, a more reasonable approach is to intervene chemically or genetically downstream of p53 by blocking the function of genes that specifically elicit the apoptotic response but which have not been associated with abnormal embryonic and postnatal development or tumor suppression in any way.
Lastly, the wide variability in the severity of the TCS phenotype highlights the existence of genetic modifiers of TCOF1/Treacle function in the pathogenesis of the characteristic craniofacial anomalies. Importantly, the TCS animal models available exhibit the same variability in penetrance and severity on different genetic backgrounds,44 thus providing invaluable resource for mapping modifiers of the TCS phenotype. The identification of both positive and negative genetic modifiers will provide further opportunities for therapeutic intervention and an improvement in the prognosis of at risk or affected individuals.
Ultimately, our long-term goal should be to identify a natural compound that could be administered before and during pregnancy, such as folic acid, that will provide measurable protection for the embryo from apoptosis without detrimental side effects during the 3 to 12-week period when the embryo is most susceptible to the development of craniofacial and other anomalies.
Acknowledgments
This research in the Trainor laboratory was supported by the Stowers Institute for Medical Research, March of Dimes (no. 6FY05-82), National Institute of Dental and Craniofacial Research (RO1 DE 016082-01) and the Hudson Foundation. Research in the Dixon laboratory was supported by the National Institutes of Health (P50 DE 016215) and the Medical Research Council, UK (G81/535).
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Fazen LE, Elmore J, Nadler HL. Mandibulo-facial dysostosis (Treacher-Collins syndrome) Am J Dis Child. 1967;113:405–410. doi: 10.1001/archpedi.1967.02090190051001. [DOI] [PubMed] [Google Scholar]
- Rovin S, Dachi SF, Borenstein DB, Cotter WB. Mandibulofacial dysostosis, a familial study of five generations. J Pediatr. 1964;65:215–221. doi: 10.1016/s0022-3476(64)80522-9. [DOI] [PubMed] [Google Scholar]
- Treacher Collins Syndrome Collaborative Group Positional cloning of a gene involved in the pathogenesis of Treacher Collins syndrome. Nat Genet. 1996;12:130–136. doi: 10.1038/ng0296-130. [DOI] [PubMed] [Google Scholar]
- Dixon J, Trainor P, Dixon MJ.Treack and Treacher Collins syndromeIn: Epstein, Erickson and Wynshaw-Boris (eds): In born Errors of Development. New York: Oxford University Press, 2008 [Google Scholar]
- Jones KL, Smith DW, Harvey MA, Hall BD, Quan L. Older paternal age and fresh gene mutation: data on additional disorders. J Pediatr. 1975;86:84–88. doi: 10.1016/s0022-3476(75)80709-8. [DOI] [PubMed] [Google Scholar]
- Dixon MJ, Marres HA, Edwards SJ, Dixon J, Cremers CW. Treacher Collins syndrome: correlation between clinical and genetic linkage studies. Clin Dysmorphol. 1994;3:96–103. [PubMed] [Google Scholar]
- Marres HA, Cremers CW, Dixon MJ, Huygen PL, Joosten FB. The Treacher Collins syndrome. A clinical, radiological, and genetic linkage study on two pedigrees. Arch Otolaryngol Head Neck Surg. 1995;121:509–514. doi: 10.1001/archotol.1995.01890050009002. [DOI] [PubMed] [Google Scholar]
- Edwards SJ, Fowlie A, Cust MP, Liu DT, Young ID, Dixon MJ. Prenatal diagnosis in Treacher Collins syndrome using combined linkage analysis and ultrasound imaging. J Med Genet. 1996;33:603–606. doi: 10.1136/jmg.33.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J, Dixon MJ. Genetic background has a major effect on the penetrance and severity of craniofacial defects in mice heterozygous for the gene encoding the nucleolar protein Treacle. Dev Dyn. 2004;229:907–914. doi: 10.1002/dvdy.20004. [DOI] [PubMed] [Google Scholar]
- Stovin JJ, Lyon JA, Jr, Clemmens RL. Mandibulofacial dysostosis. Radiology. 1960;74:225–231. doi: 10.1148/74.2.225. [DOI] [PubMed] [Google Scholar]
- Phelps PD, Poswillo D, Lloyd GA. The ear deformities in mandibulofacial dysostosis (Treacher Collins syndrome) Clin Otolaryngol. 1981;6:15–28. doi: 10.1111/j.1365-2273.1981.tb01782.x. [DOI] [PubMed] [Google Scholar]
- Dixon MJ, Read AP, Donnai D, Colley A, Dixon J, Williamson R. The gene for Treacher Collins syndrome maps to the long arm of chromosome 5. Am J Hum Genet. 1991;49:17–22. [PMC free article] [PubMed] [Google Scholar]
- Chemke J, Mogilner BM, Ben-Itzhak I, Zurkowski L, Ophir D. Autosomal recessive inheritance of Nager acrofacial dysostosis. J Med Genet. 1988;25:230–232. doi: 10.1136/jmg.25.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin RJ, Cohen MM, Levin LS. Syndromes of the Head and Neck. Oxford, UK: Oxford University Press; 1990. [Google Scholar]
- Allanson JE, McGillivray BC. Familial clefting syndrome with ectropion and dental anomaly--without limb anomalies. Clin Genet. 1985;27:426–429. doi: 10.1111/j.1399-0004.1985.tb02288.x. [DOI] [PubMed] [Google Scholar]
- Ogilvy-Stuart AL, Parsons AC. Miller syndrome (postaxial acrofacial dysostosis): further evidence for autosomal recessive inheritance and expansion of the phenotype. J Med Genet. 1991;28:695–700. doi: 10.1136/jmg.28.10.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt EM, Travis F, Lefebvre A, Munro IR. Psychosocial adjustment of 20 patients with Treacher Collins syndrome before and after reconstructive surgery. Br J Plast Surg. 1987;40:605–609. doi: 10.1016/0007-1226(87)90155-x. [DOI] [PubMed] [Google Scholar]
- Poswillo D. The pathogenesis of the Treacher Collins syndrome (mandibulofacial dysostosis) Br J Oral Surg. 1975;13:1–26. doi: 10.1016/0007-117x(75)90019-0. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Smiley SJ, Speight HS, Jarvis BE. Mandibulofacial dysostosis (Treacher Collins syndrome): a new proposal for its pathogenesis. Am J Med Genet. 1987;27:359–372. doi: 10.1002/ajmg.1320270214. [DOI] [PubMed] [Google Scholar]
- Sulik K, Cook C, Webster W. Teratogens and craniofacial malformations: relationships to cell death. Development (Cambridge, England) 1988;103:213–232. doi: 10.1242/dev.103.Supplement.213. [DOI] [PubMed] [Google Scholar]
- Wiley MJ, Cauwenbergs P, Taylor IM. Effects of retinoic acid on the development of the facial skeleton in hamsters: early changes involving cranial neural crest cells. Acta Anat (Basel) 1983;116:180–192. doi: 10.1159/000145741. [DOI] [PubMed] [Google Scholar]
- Herring SW, Rowlatt UF, Pruzansky S. Anatomical abnormalities in mandibulofacial dysostosis. Am J Med Genet. 1979;3:225–229. doi: 10.1002/ajmg.1320030303. [DOI] [PubMed] [Google Scholar]
- Dixon J, Jones NC, Sandell LL, et al. Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc Natl Acad Sci USA. 2006;103:13403–13408. doi: 10.1073/pnas.0603730103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J, Edwards SJ, Anderson I, Brass A, Scambler PJ, Dixon MJ. Identification of the complete coding sequence and genomic organization of the Treacher Collins syndrome gene. Genome Res. 1997;7:223–234. doi: 10.1101/gr.7.3.223. [DOI] [PubMed] [Google Scholar]
- Wise CA, Chiang LC, Paznekas WA, et al. TCOF1 gene encodes a putative nucleolar phosphoprotein that exhibits mutations in Treacher Collins Syndrome throughout its coding region. Proc Natl Acad Sci USA. 1997;94:3110–3115. doi: 10.1073/pnas.94.7.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh KL, Dixon J, Dixon MJ. Mutations in the Treacher Collins syndrome gene lead to mislocalization of the nucleolar protein treacle. Hum Mol Genet. 1998;7:1795–1800. doi: 10.1093/hmg/7.11.1795. [DOI] [PubMed] [Google Scholar]
- Winokur ST, Shiang R. The Treacher Collins syndrome (TCOF1) gene product, treacle, is targeted to the nucleolus by signals in its C-terminus. Hum Mol Genet. 1998;7:1947–1952. doi: 10.1093/hmg/7.12.1947. [DOI] [PubMed] [Google Scholar]
- Isaac C, Marsh KL, Paznekas WA, et al. Characterization of the nucleolar gene product, treacle, in Treacher Collins syndrome. Mol Biol Cell. 2000;11:3061–3071. doi: 10.1091/mbc.11.9.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez BC, Henning D, So RB, Dixon J, Dixon MJ. The Treacher Collins syndrome (TCOF1) gene product is involved in ribosomal DNA gene transcription by interacting with upstream binding factor. Proc Natl Acad Sci USA. 2004;101:10709–10714. doi: 10.1073/pnas.0402492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano T, Yanagida M, Yamauchi Y, Shinkawa T, Isobe T, Takahashi N. Proteomic analysis of human Nop56p-associated pre-ribosomal ribonucleoprotein complexes. Possible link between Nop56p and the nucleolar protein treacle responsible for Treacher Collins syndrome. J Biol Chem. 2003;278:34309–34319. doi: 10.1074/jbc.M304304200. [DOI] [PubMed] [Google Scholar]
- Jones NC, Lynn ML, Gaudenz K, et al. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat Med. 2008;14:125–133. doi: 10.1038/nm1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd HP, Fridell RA, Benson RE, Hua J, Cullen BR. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol Cell Biol. 1996;16:4207–4214. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emes RD, Ponting CP. A new sequence motif linking lissencephaly, Treacher Collins and oral-facial-digital type 1 syndromes, microtubule dynamics and cell migration. Hum Mol Genet. 2001;10:2813–2820. doi: 10.1093/hmg/10.24.2813. [DOI] [PubMed] [Google Scholar]
- Feng Y, Olson EC, Stukenberg PT, Flanagan LA, Kirschner MW, Walsh CA. LIS1 regulates CNS lamination by interacting with mNudE, a central component of the centrosome. Neuron. 2000;28:665–679. doi: 10.1016/s0896-6273(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Shionoya A, Ishida M, et al. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Jurkovic D, Gruboeck K, Campbell S. Ultrasound features of normal early pregnancy development. Curr Opin Obstet Gynecol. 1995;7:493–504. [PubMed] [Google Scholar]
- Ghi T, Perolo A, Banzi C, et al. Two-dimensional ultrasound is accurate in the diagnosis of fetal craniofacial malformation. Ultrasound Obstet Gynecol. 2002;19:543–551. doi: 10.1046/j.1469-0705.2002.00721.x. [DOI] [PubMed] [Google Scholar]
- Glick PL, Harrison MR, Golbus MS, et al. Management of the fetus with congenital hydronephrosis II: prognostic criteria and selection for treatment. J Pediatr Surg. 1985;20:376–387. doi: 10.1016/s0022-3468(85)80223-2. [DOI] [PubMed] [Google Scholar]
- Dodson TB, Schmidt B, Longaker MT, Kaban LB. Fetal cleft lip repair in rabbits: postnatal facial growth after repair. J Oral Maxillofac Surg. 1991;49:603–611. doi: 10.1016/0278-2391(91)90342-j. [DOI] [PubMed] [Google Scholar]
- Stern M, Schmidt B, Dodson TB, Stern R, Kaban LB.Fetal cleft lip repair in rabbits: histology and role of hyaluronic acid J Oral Maxillofac Surg 199250263–268.discussion 269. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Aylon Y, Oren M. Living with p53, dying of p53. Cell. 2007;130:597–600. doi: 10.1016/j.cell.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Dixon J, Brakebusch C, Fassler R, Dixon MJ. Increased levels of apoptosis in the prefusion neural folds underlie the craniofacial disorder, Treacher Collins syndrome. Hum Mol Genet. 2000;9:1473–1480. doi: 10.1093/hmg/9.10.1473. [DOI] [PubMed] [Google Scholar]
Further Reading
- Dixon J, Dixon MJ.TCOF1/Treacle and the Treacher Collins Syndromein Esptein, Erickson and Wynshaw-Boris (eds):Inborn Errors of Development – the Molecular Basis of Clinical Disorders of Morphogenesis New York: Oxford University Press; 2004 [Google Scholar]
- Johnston M, Bronsky PT.Craniofacial Embryogenesis – Abnormal developmental mechanismsin: Mooney and Siegel (eds): Understanding Craniofacial Anomalies; Etiopathogenesis of Craniosynostoses and Facial Clefting New York: Wiley-Liss; 2002 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.