Abstract
Noonan syndrome (NS) and cardio-facio-cutaneous syndrome (CFCS) are related developmental disorders caused by mutations in genes encoding various components of the RAS-MAPK signaling cascade. NS is associated with mutations in the genes PTPN11, SOS1, RAF1, or KRAS, whereas CFCS can be caused by mutations in BRAF, MEK1, MEK2, or KRAS. The NS phenotype is rarely accompanied by multiple giant cell lesions (MGCL) of the jaw (Noonan-like/MGCL syndrome (NL/MGCLS)). PTPN11 mutations are the only genetic abnormalities reported so far in some patients with NL/MGCLS and in one individual with LEOPARD syndrome and MGCL. In a cohort of 75 NS patients previously tested negative for mutations in PTPN11 and KRAS, we detected SOS1 mutations in 11 individuals, four of whom had MGCL. To explore further the relevance of aberrant RAS-MAPK signaling in syndromic MGCL, we analyzed the established genes causing CFCS in three subjects with MGCL associated with a phenotype fitting CFCS. Mutations in BRAF or MEK1 were identified in these patients. All mutations detected in these seven patients with syndromic MGCL had previously been described in NS or CFCS without apparent MGCL. This study demonstrates that MGCL may occur in NS and CFCS with various underlying genetic alterations and no obvious genotype–phenotype correlation. This suggests that dysregulation of the RAS-MAPK pathway represents the common and basic molecular event predisposing to giant cell lesion formation in patients with NS and CFCS rather than specific mutation effects.
Keywords: Noonan syndrome, cardio-facio-cutaneous syndrome, multiple giant cell lesions, Noonan-like/multiple giant cell lesion syndrome, RAS-MAPK signaling cascade
Introduction
Noonan syndrome (NS; MIM 163950) is an autosomal dominant developmental disorder characterized by congenital heart defects, facial anomalies, short stature, webbed neck, chest deformity, developmental delay of variable degree, and cryptorchidism in males.1, 2 Several distinct entities show phenotypic similarities to NS, including LEOPARD syndrome (LS; MIM 151100), cardio-facio-cutaneous syndrome (CFCS; MIM 115150), and Costello syndrome (CS; MIM 218040). This group of clinically overlapping disorders is caused by mutations in genes encoding various components of the RAS-MAPK pathway, which relays signals from activated cell surface receptors to the nucleus. NS can be caused by mutations in the genes PTPN11, SOS1, RAF1 or KRAS,3, 4, 5, 6, 7, 8 whereas CFCS is associated with mutations in BRAF, MEK1, MEK2 or KRAS.9, 10 HRAS mutations have been identified in the vast majority of patients with Costello syndrome11, 12 and specific PTPN11 mutations are responsible for LS.13, 14
Giant cell lesions (GCL) are benign tumor-like lesions most frequently affecting the jaws but also occurring in other bones or soft tissues. They consist of multinucleated giant cells in a background of fibrous connective tissue with abundant spindle-shaped mononucleated cells.15 The pathogenesis of GCL formation is incompletely understood. There is evidence that the mononucleated (osteoblast-like) cell population represents proliferating tumor cells that produce cytokines inducing the maturation of a subset of mononuclear phagocytes into osteoclast-like giant cells.15 GCL can be multilocular, and extensive involvement of the jaws can lead to the clinical picture of cherubism (MIM 118400), which can be caused by mutations of SH3BP2,16 a gene encoding for an adapter protein involved in intracellular signaling in hematopoietic and hematopoietic lineage-derived cells.
Multiple giant cell lesions (MGCL) have repeatedly been observed in patients with clinical features of NS. The term Noonan-like/MGCL syndrome (NL/MGCLS; MIM 163955) was coined for this association.17, 18 Once thought to be a separate entity, it is now rather considered a variant of the NS spectrum.19, 20, 21, 22 This notion was supported by the finding of PTPN11 mutations in a number of patients with NL/MGCLS or a related phenotype.20, 21, 22 Of note, these reports identified different PTPN11 mutations (p.D106A, p.F285L, p.N308S, p.A461T), which had previously been observed to occur in subjects with NS or LS.20, 21, 22 Although the relatively small number of cases analyzed so far does not allow one to ascertain whether specific PTPN11 mutations are preferentially associated with the development of MGCL, the finding that the same changes found in NL/MGCLS patients occur in NS patients without MGCL indicates that the heterozygous condition for a PTPN11 mutation is not sufficient to produce these lesions. The available genetic data support the view that NL/MGCLS, similar to NS, is genetically heterogeneous,22 suggesting that other genes coding for transducers participating in the RAS-MAPK pathway might be involved in MGCL pathogenesis.
Herein, we report that syndromic MGCL may be caused by mutations in various genes encoding other components of the RAS-MAPK signaling cascade.
Patients and methods
The study population comprised a cohort of 75 patients with a clinical diagnosis of NS, 4 of whom had MGCL. This cohort included 49 NS cases originally studied by Musante et al,23 as well as newly ascertained cases. All of them had previously tested negative for PTPN11 and KRAS mutations. The group of patients with syndromic MGCL was completed by three individuals displaying MGCL associated with a phenotype in keeping with or suggestive of CFCS. The patients were screened for mutations in the genes SOS1 and BRAF, MEK1 and MEK2, respectively.
The study was approved by the Review Boards of the Universities of Erlangen and Berlin, and Children's Hospital of Eastern Ontario, Ottawa. Written informed consent was obtained from all patients or their legal guardians for DNA analysis, publication of data, and for the photographs presented here.
DNA extracted from blood cells was used for molecular genetic testing. Mutational screening was carried out by bidirectional direct sequencing using the BigDye Terminator Sequencing Kit v2.1 (Applied Biosystems) and an automated capillary sequencer (ABI 3730, Applied Biosystems) as described previously.24 SOS1 screening was performed on exons 3, 6–8, and 10–16, as these exons contain all nucleotides reported previously to be mutated in patients with NS.4, 5, 24 Similarly, BRAF mutation analysis was performed on exons 6 and exons 11–16, whereas screening of the MEK1 and MEK2 genes was restricted to exons 2 and 3. SH3BP2 analyses were performed as published.16 Primer pairs and PCR conditions are available on request.
Results
Mutations in the SOS1 gene were identified in 11 of the 75 index cases from the PTPN11 and KRAS mutation-negative NS patient group, thus giving an SOS1 mutation frequency of 14.7% in this cohort. All mutations had previously been documented in NS.4, 5, 24 Among the NS patients tested positive for an SOS1 mutation were the four individuals with MGCL (Table 1). Molecular screening performed on the additional three syndromic MGCL cases displaying a phenotype fitting CFCS or suggestive of this condition identified a heterozygous BRAF or MEK1 gene mutation in all individuals (Table 1). All three mutations were de novo. All of the three patients exhibited moderate-to-severe cognitive defects, and two of them showed typical ectodermal changes (Table 2). Taken together, all subjects with a diagnosis of NS or CFCS with MGCL were found to carry a mutated SOS1, BRAF, or MEK1 allele. All mutations were missense and had previously been reported in subjects with NS and CFCS, respectively, without apparent MGCL.4, 5, 10, 24, 25
Table 1. SOS1, BRAF and MEK1 gene mutations detected in the syndromic MGCL and NS cohorts.
| Disorder and number of cases | Gene | Nucleotide change | Amino acid change |
|---|---|---|---|
| NS with MGCL | |||
| 1 | SOS1 | c.1297G>A | p.E433K |
| 1 | SOS1 | c.1656G>T | p.R552S |
| 2 | SOS1 | c.2536G>A | p.E846K |
| CFCS with MGCL | |||
| 2 | BRAF | c.770A>G | p.Q257R |
| 1 | MEK1 | c.389A>G | p.Y130C |
| NS | |||
| 1 | SOS1 | c.806T>G | p.M269R |
| 1 | SOS1 | c.1649T>C | p.L550P |
| 5a | SOS1 | c.1654A>G | p.R552G |
Including one family transmitting the trait.
Table 2. Clinical findings in patients with syndromic MGCL.
| Patient ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Age of last examination (years) | 28 | 15 | 9 | 22 | 24 | 7 | 10 |
| Sex | F | F | M | M | F | M | M |
| Age at diagnosis of MGCL (years) | 11 | 10 | 8 | 13 | 14 | 5 | 8 |
| Height SDS | 10th centile | <3rd centile | 3rd centilea | 10th centile | <5th centile | <5th centile | 1st centile |
| Heart defect | PS, ASD | PS | PS, MI | ASD, vena cava stenosis | ASD, mild HCM, cleft mitral valve | PS | Mild PS, mild HCM |
| Pectus deformation | + | + | + | + | − | + | − |
| Webbed neck | + | + | + | + | − | − | − |
| Osteopenia | − | − | − | + | − | − | − |
| Curly hair | + | − | + | + | + | + | − |
| Hyperkeratosis | − | − | − | − | + | + | + |
| Mental retardation | − | − | − | − | Severe | Moderate–severe | Moderate–severe |
| Hearing impairment | + | − | − | − | − | − | + |
| Ocular | Ptosis | Ptosis | Ptosis, strabismus, myopia | Ptosis, hypertelorism | Ptosis, strabismus, nystagmus | Ptosis | Ptosis, hypertelorism |
| Other | Anal atresia | − | Hypothyroidism | Kyphoscoliosis | Multiple nevi | Hydronephrosis | Epilepsy |
| Mutation | SOS1 p.R552S | SOS1 p.E433K | SOS1 p.E846K | SOS1 p.E846K | BRAF p.Q257R | BRAF p.Q257R | MEK1 p.Y130C |
Abbreviations: ASD, atrial septal defect; HCM, hypertrophic cardiomyopathy; MI mitral insufficiency; PS, pulmonary valve stenosis.
Patient received growth hormone treatment.
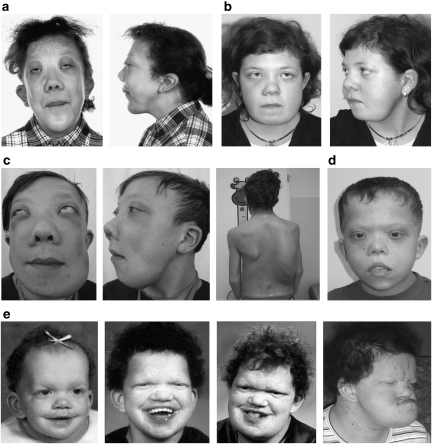
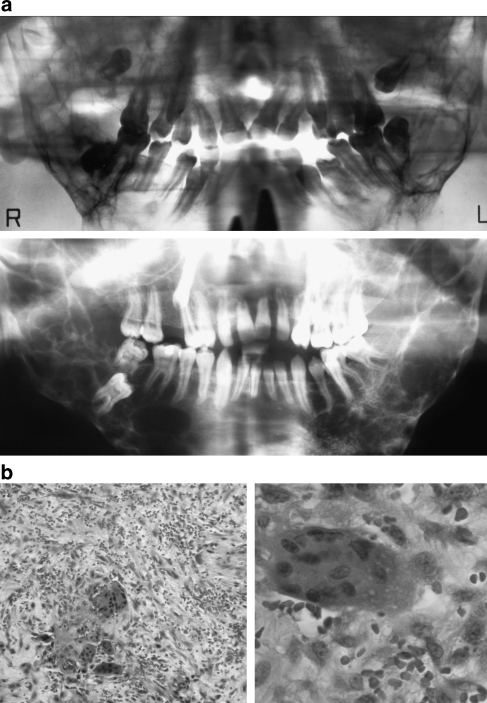
In all but one patient affected by MGCLs, the diagnosis of NS/CFCS had been made clinically in infancy or childhood before the appearance of disfiguring facial bone involvement. In patient 4, who first came to medical attention because of his mandibular tumescence, an initial diagnosis of cherubism was made, whereas it was noticed later that his additional features were compatible with NS. Enlargement of the jaws was recognized at the age of 5–14 years in this cohort and has been slowly progressive in each patient. In the three adult patients (patients 1, 4, and 5), GCLs have still been growing, in contrast to cherubism, where a regression of GCLs is usually observed after puberty.15 All patients except for patient 7 showed bilateral involvement of the mandible. All patients had undergone facial bone surgery and the typical histological appearance of GCL was confirmed. Detailed clinical characterization of these subjects is reported in Table 2. Among them, patients 1, 4, and 5 exhibited the most severe bony changes resembling the clinical picture of cherubism (Figure 1a, c and e). Orthopantograms of patients 1 and 4 and histology photos of GCLs in patient 7 are shown in Figure 2. SH3BP2 mutation analysis had been performed in patients 1 and 4, and no mutation was identified.
Figure 1.
Clinical features in patients with syndromic MGCLS. Severe involvement of the jaws resembling cherubism is present in patients 1 (a), 4 (c), and 5 (e). The second also shows severe scoliosis (c). Milder expression in patients 2 (b) and 7 (d). Development of the facial shape in patient 5 (e). (Patient are numbered according to Table 2.)
Figure 2.
(a) The radiographs of patients 1 and 4 show bilateral extensive radiolucent lesions in the posterior mandible. (b) The histology pictures of patient 7 show multinucleated giant cells within a fibrous stroma. (HE stain, × 100 and × 400 original magnification. Patients are numbered according to Table 2.)
Discussion
Noonan-like/MGCL syndrome was previously found to be associated with PTPN11 mutations.20, 21, 22, 26 This is the first study that describes the association between MGCL and mutations in other genes encoding components of the RAS-MAPK pathway. The observed mutations are presumed to lead to increased/dysregulated signaling.27, 28, 29 Taken together, the findings in previous reports and the results of this study suggest that various genetic alterations leading to dysregulation of the RAS-MAPK pathway can contribute to the development of MGCL. This is consistent with the observation that MGCL are also a possible manifestation of neurofibromatosis type 1 (NF1).30, 31, 32, 33 NF1, the gene mutated in NF1, encodes neurofibromin that acts as a GTPase-activating protein (GAP) for RAS. Loss of GAP activity leads to constitutive activation of RAS and RAS-mediated signaling, which is functionally similar to the presumed effect of activating mutations in PTPN11, SOS1, BRAF, and MEK1.27, 28, 29
We also demonstrate for the first time that syndromic MGCLS is not only associated with mutations that are typical of NS or LS. The same bony lesions can be present in patients who have clinical features consistent with or suggestive of CFCS and are heterozygous for mutations in genes known to be mutated in this developmental disorder (cases 5–7; Table 2). Apart from the MGCL, the clinical phenotype of these patients does not differ from that characterizing NS and CFCS patients without MGCL. These findings emphasize that NL/MGCLS should no longer be regarded as a separate entity. Instead, development of MGCL should be considered as a rare but typical complication that may occur in patients with any of the disorders having in common the dysregulation of RAS-MAPK signaling. The incidence of MGCL in patients with NS or CFCS is not known and has not been studied systematically. Whereas the unexpectedly high prevalence of MGCL within the relatively small cohort of SOS1 mutation-positive subjects with NS included in this study is most likely due to chance, it is possible that single and small lesions in the jaws might represent relatively common events in these conditions, with only extensive growth and multilocular occurrence most likely to be noticed clinically. We also consider the possibility that MGCL may occur in patients with NS/CFCS and mutations in any of the other known as well as in hitherto unknown genes. An orthopantogram should be recommended in patients with NS/CFCS and progressive facial coarsening.
Consequently, we postulate that dysregulation of the RAS-MAPK pathway represents the common and basic molecular event predisposing to MGCL formation in patients with NS, LS, CFCS, and NF1. It is not clear whether specific mutations are associated with an increased risk for MGCL development, whereas others are not. Certain mutations have been observed repeatedly in patients affected by NS/CFCS with MGCL, such as PTPN11 mutations p.D106A and p.F285L,4, 5, 22, 26 the SOS1 mutation p.E846K, and the BRAF mutation p.Q257R (this article), but the number of patients with confirmed mutations is still too small to draw definite conclusions. Of note, the same changes have been found also in adult patients without apparent MGCL, indicating that these mutations are not sufficient to produce the tumor-like lesion. We therefore speculate that additional factors (eg, modifier genes, epigenetic factors, or somatic ‘second hits') contribute to the manifestation of MGCL in patients with NS, LS, CFCS, or NF1. The observations of a ‘progressing phenotype' in individual patients21 and of multiple family members affected by an NS-like disorder but being discordant for the presence of MGCL19 support this hypothesis.
The association of MGCL with disorders of the RAS-MAPK pathway further raises the speculation that somatic mutations in components of this pathway might play a role in the pathogenesis of nonsyndromic MGCL. It was previously shown that a specific type of lymphoproliferative disorder, juvenile myelomonocytic leukemia (JMML), is particularly related to mutations leading to upregulated RAS-MAPK signaling, either as germline events associated with NS or NF1 or as somatic mutations in nonsyndromic cases.34, 35 Likewise, MGCL might represent another tumor-like lesion specifically linked to abnormal RAS signaling. As osteoclasts originate from hematopoietic cells similar to the myeloid precursors that underlie JMML, a link between these different kinds of tumors seems reasonable. Ueki et al36 have recently shown that myeloid cells from mice expressing the cherubism-causing P416R SH3BP2 mutant show increased responses to M-CSF and RANKL stimulation and form macrophages and osteoclasts through mechanisms that include increased ERK activation. Inappropriate ERK activation in these cells may therefore represent the common pathogenetic mechanism that underlies the development of MGCL.
In summary, we show that MGCL may occur in patients with NS and CFCS with different underlying molecular changes. We speculate that development of MGCL is related to RAS-MAPK pathway dysregulation as the basic underlying mechanism rather than specific mutation effects. These aspects argue against the existence of an NL/MGCLS as a separate entity. The findings of this study should prompt molecular investigations in isolated GCL and nonsyndromic MGCL.
Acknowledgments
We thank the patients and their families for participating in this study and to share their personal information. The study was supported by grants from the German Research Foundation (DFG) to MZ (Ze 524/4-1), and Telethon-Italy to MT (GGP07115). VC was supported by a fellowship from Associazione ONLUS ‘Morgan Di Gianvittorio per la cura e la ricerca nei tumori e leucemie in età pediatrica'.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Noonan JA. Hypertelorism with Turner phenotype. A new syndrome with associated congenital heart disease. Am J Dis Child. 1968;116:373–380. doi: 10.1001/archpedi.1968.02100020377005. [DOI] [PubMed] [Google Scholar]
- Allanson JE. Noonan syndrome. J Med Genet. 1987;24:9–13. doi: 10.1136/jmg.24.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Mehler EL, Goldberg R, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- Roberts AE, Araki T, Swanson KD, et al. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet. 2007;39:70–74. doi: 10.1038/ng1926. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Pennacchio LA, Zhao C, et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat Genet. 2007;39:75–79. doi: 10.1038/ng1939. [DOI] [PubMed] [Google Scholar]
- Razzaque MA, Nishizawa T, Komoike Y, et al. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat Genet. 2007;39:1013–1017. doi: 10.1038/ng2078. [DOI] [PubMed] [Google Scholar]
- Schubbert S, Zenker M, Rowe SL, et al. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38:331–336. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- Pandit B, Sarkozy A, Pennacchio LA, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007–1012. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- Niihori T, Aoki Y, Narumi Y, et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet. 2006;38:294–296. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Tetsu O, Tidyman WE, et al. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311:1287–1290. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Niihori T, Kawame H, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- Zenker M, Lehmann K, Schulz AL, et al. Expansion of the genotypic and phenotypic spectrum in patients with KRAS germline mutations. J Med Genet. 2007;44:131–135. doi: 10.1136/jmg.2006.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legius E, Schrander-Stumpel C, Schollen E, Pulles-Heintzberger C, Gewillig M, Fryns JP. PTPN11 mutations in LEOPARD syndrome. J Med Genet. 2002;39:571–574. doi: 10.1136/jmg.39.8.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digilio MC, Conti E, Sarkozy A, et al. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet. 2002;71:389–394. doi: 10.1086/341528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange J, van Maarle MC, van den Akker HP, Redeker EJ. A new mutation in the SH3BP2 gene showing reduced penetrance in a family affected with cherubism. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:378–381. doi: 10.1016/j.tripleo.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Tiziani V, Santanna C, et al. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat Genet. 2001;28:125–126. doi: 10.1038/88832. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Ruvalcaba RHA, Graham CB, Harrison MT, Morgan AF. A new syndrome simulating the Noonan syndrome, the Leopard syndrome, and hyperparathyroidism. Syndrome Ident. 1974;2:14–17. [Google Scholar]
- Cohen MM, Jr, Gorlin RJ. Noonan-like/multiple giant cell lesion syndrome. Am J Med Genet. 1991;40:159–166. doi: 10.1002/ajmg.1320400208. [DOI] [PubMed] [Google Scholar]
- Bertola DR, Kim CA, Pereira AC, et al. Are Noonan syndrome and Noonan-like/multiple giant cell lesion syndrome distinct entities. Am J Med Genet. 2001;98:230–234. doi: 10.1002/1096-8628(20010122)98:3<230::aid-ajmg1080>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Kalidas K, Shaw A, et al. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet. 2002;70:1555–1563. doi: 10.1086/340847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkozy A, Obregon MG, Conti E, et al. A novel PTPN11 gene mutation bridges Noonan syndrome, multiple lentigines/LEOPARD syndrome and Noonan-like/multiple giant cell lesion syndrome. Eur J Hum Genet. 2004;12:1069–1072. doi: 10.1038/sj.ejhg.5201290. [DOI] [PubMed] [Google Scholar]
- Lee JS, Tartaglia M, Gelb BD, et al. Phenotypic and genotypic characterisation of Noonan-like/multiple giant cell lesion syndrome. J Med Genet. 2005;42:e11. doi: 10.1136/jmg.2004.024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musante L, Kehl HG, Majewski F, et al. Spectrum of mutations in PTPN11 and genotype-phenotype correlation in 96 patients with Noonan syndrome and five patients with cardio-facio-cutaneous syndrome. Eur J Hum Genet. 2003;11:201–206. doi: 10.1038/sj.ejhg.5200935. [DOI] [PubMed] [Google Scholar]
- Zenker M, Horn D, Wieczorek D, et al. SOS1 is the second most common Noonan gene but plays no major role in cardio-facio-cutaneous syndrome. J Med Genet. 2007;44:651–656. doi: 10.1136/jmg.2007.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow A, Steinemann D, Gohring G, et al. Clonal duplication of a germline PTPN11 mutation due to acquired uniparental disomy in acute lymphoblastic leukemia blasts from a patient with Noonan syndrome. Leukemia. 2007;21:1303–1305. doi: 10.1038/sj.leu.2404651. [DOI] [PubMed] [Google Scholar]
- Jafarov T, Ferimazova N, Reichenberger E. Noonan-like syndrome mutations in PTPN11 in patients diagnosed with cherubism. Clin Genet. 2005;68:190–191. doi: 10.1111/j.1399-0004.2005.00475.x. [DOI] [PubMed] [Google Scholar]
- Gelb BD, Tartaglia M. Noonan syndrome and related disorders: dysregulated RAS-mitogen activated protein kinase signal transduction. Hum Mol Genet. 2006;15 Spec No 2:R220–R226. doi: 10.1093/hmg/ddl197. [DOI] [PubMed] [Google Scholar]
- Schubbert S, Bollag G, Shannon K. Deregulated Ras signaling in developmental disorders: new tricks for an old dog. Curr Opin Genet Dev. 2007;17:15–22. doi: 10.1016/j.gde.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kratz CP, Niemeyer CM, Zenker M. An unexpected new role of mutant Ras: perturbation of human embryonic development. J Mol Med. 2007;85:227–235. doi: 10.1007/s00109-006-0135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz H, Petersen D, Heiss E, Duffner F, Meyermann R. Giant cell tumor of the occipital bone in a case of von Recklinghausen neurofibromatosis. Clin Neuropathol. 1996;15:226–230. [PubMed] [Google Scholar]
- Ruggieri M, Pavone V, Polizzi A, et al. Unusual form of recurrent giant cell granuloma of the mandible and lower extremities in a patient with neurofibromatosis type 1. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:67–72. doi: 10.1016/s1079-2104(99)70297-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Tello FJ, Manjon-Luengo P, Martin-Perez M, Montes-Moreno S. Cherubism associated with neurofibromatosis type 1, and multiple osteolytic lesions of both femurs: a previously undescribed association of findings. Skeletal Radiol. 2005;34:793–798. doi: 10.1007/s00256-005-0938-3. [DOI] [PubMed] [Google Scholar]
- van Capelle CI, Hogeman PH, van der Sijs-Bos CJ, et al. Neurofibromatosis presenting with a cherubism phenotype. Eur J Pediatr. 2007;166:905–909. doi: 10.1007/s00431-006-0334-6. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Niemeyer CM, Fragale A, et al. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet. 2003;34:148–150. doi: 10.1038/ng1156. [DOI] [PubMed] [Google Scholar]
- Kratz CP, Niemeyer CM, Castleberry RP, et al. The mutational spectrum of PTPN11 in juvenile myelomonocytic leukemia and Noonan syndrome/myeloproliferative disease. Blood. 2005;106:2183–2185. doi: 10.1182/blood-2005-02-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Lin CY, Senoo M, et al. Increased myeloid cell responses to M-CSF and RANKL cause bone loss and inflammation in SH3BP2 ‘cherubism' mice. Cell. 2007;128:71–83. doi: 10.1016/j.cell.2006.10.047. [DOI] [PubMed] [Google Scholar]