Abstract
Nephronophthisis (NPHP) is an autosomal recessive kidney disorder characterized by chronic tubulointerstitial nephritis and leading to end-stage renal failure. NPHP as a renal entity is often part of a multisystem disorder and has been associated with many syndromes including Joubert syndrome (and related disorders) and Senior–Loken syndrome. Recent molecular genetic advances have allowed identification of several genes underlying NPHP. Most of these genes express their protein products, named nephrocystins, in primary cilial/basal body structures. Some nephrocystins are part of adherens junction and focal adhesion kinase protein complexes. This shared localization suggests that common pathogenic mechanisms within the kidney underlie this disease. Functional studies implicate nephrocystins in planar cell polarity pathways, which may be crucial for renal development and maintenance of tubular architecture.
Keywords: primary cilia, collecting duct, planar-cell polarity, urine concentrating defect, tubulointerstitial nephritis
In brief
Nephronophthisis (NPHP) is an autosomal recessive kidney disease leading to end-stage renal failure in children and young adults.
Key histological findings in the kidney are tubulointerstitial fibrosis, tubular dilatation and cyst formation and tubular atrophy.
NPHP is often a feature of a multisystem disease that may include retinal dystrophy (Senior–Loken Syndrome) and cerebello-ocular-renal syndromes (Joubert syndrome and related diseases (JSRD)).
NPHP may present with an early decrease in urinary concentration
End-stage renal failure (ESRF) typically occurs during early teenage years, with the exception of the rare infantile forms, where there is ESRF before 5 years of age.
Molecular genetics now may allow easy detection of the most common mutations (involving NPHP1 and accounting for 25% of all cases).
NPHP is a ‘ciliopathy' as evidence to date implicates the primary renal cilium and basal body apparatus in the pathogenesis of NPHP.
Patients need regular monitoring of renal and liver function, eye examinations and preparation for renal transplantation, which is the treatment of choice for the renal failure that invariably ensues.
Introduction
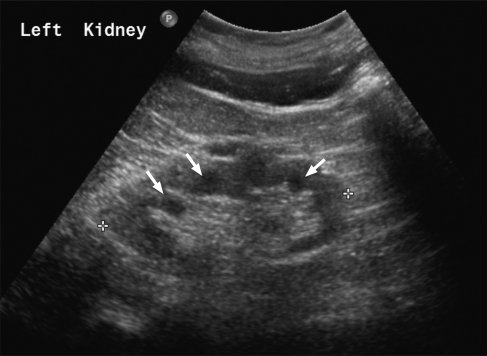
Nephronophthisis (NPHP) is an autosomal recessively inherited renal disorder, which leads to progressive renal failure, usually within the first 3 decades of life.1 Nephronophthisis literally means ‘disappearance of nephrons'. Typical ultrasound features include normal or reduced renal size, loss of corticomedullary differentiation and corticomedullary cysts (Figure 1). Renal biopsy findings include tubular atrophy, interstitial fibrosis and tubular basement membrane defects, including abrupt transition between thickening and attenuation or disintegration.2, 3 A rare form of NPHP may lead to end-stage renal failure (ESRF) within 5 years of age and is termed infantile NPHP.4 This differs from typical NPHP in that there is moderate renal enlargement, histological changes that include cortical microcysts, cystic dilatation of Bowman's spaces and lack of tubular basement membrane disruption.
Figure 1.
Ultrasound scan features of nephronophthisis. Renal ultrasound scan demonstrating corticomedullary cysts, some of which are arrowed.
NPHP is often part of a spectrum of multisystem disease and may not be detected unless appropriate investigations on relevant systems are performed. These disease associations form a very heterogeneous group (Table 1). The most commonly associated syndrome is retinal dystrophy and retinal degeneration leading to blindness (Senior–Loken syndrome).1 Other associations include Joubert syndrome and related diseases (JSRD, reviewed in reference5), which often involves a cerebellar, retinal and renal phenotype referred to as CORS (cerebello-oculo-renal syndrome). Apart from these, a whole variety of syndromes have been reported in association with NPHP (Table 1).
Table 1. Syndromes which may exhibit nephronophthisis or are associated with mutations in NPHP genes.
| Syndrome | Key features |
|---|---|
| Joubert syndrome and related disorders | Cerebellar vermis aplasia/hypoplasia |
| Cogan syndrome | Oculomotor apraxia |
| Senior–Loken syndrome | Retinitis pigmentosa |
| Meckel–Gruber syndrome | Occipital meningoencephalocoele, cystic kidneys and postaxial polydactyly |
| RHYNS syndrome | Retinitis pigmentosa, hypopituitarism, nephronophthisis, skeletal dysplasia |
| Boichis syndrome | Liver fibrosis, biliary duct proliferation |
| Mainzer–Saldino syndrome or conorenal syndrome | Cone-shaped epiphyses |
| Jeune syndrome or asphyxiating thoracic dystrophy syndrome | Short ribs |
| Sensenbrenner syndrome or cranioectodermal dysplasia | Skeletal dysplasia |
| Ellis van Creveld | Ectodermal dysplasia |
| Alstrom | Retinal dystrophy, hearing impairment, obesity, type 2 diabetes mellitus |
| Arima syndrome | Cerebro-oculo-hepato-renal syndrome |
NPHP has been reported worldwide, yet the incidence varies. A Canadian study reported an incidence of 1 in 50 000 live births,6 whereas the incidence in the United States of America was estimated to be 9 per 8.3 million.7 A more recent European study reported an incidence of NPHP as 1 in 61 800 live births.8 However, as NPHP may present in adults with late enuresis and renal failure,9 these figures may be an underestimate.
Clinical overview
Core diagnostic criteria
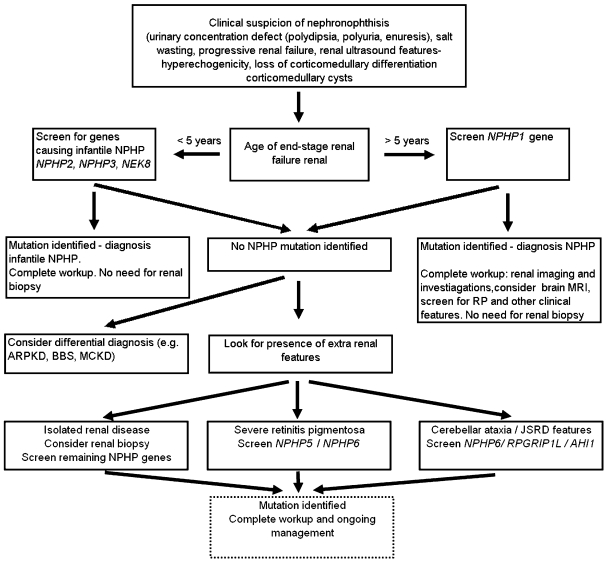
NPHP is genetically and clinically heterogeneous. Traditionally, NPHP has been subdivided into infantile, juvenile and adolescent forms, based on the age of onset of renal failure. It remains useful to distinguish the much rarer infantile NPHP from the more typical (non-infantile) forms of NPHP, to allow a targeted approach to diagnosis and molecular testing (Figure 2).
Figure 2.
Diagnostic algorithm for NPHP. Where there is clinical or radiological suspicion of NPHP, the NPHP1 gene should be screened first if onset of end-stage renal failure is greater than 5 years of age. NPHP1 mutations account for ∼25% of cases of NPHP. Infantile NPHP is rare (<1% of cases) but should be suspected, and the known genes screened, if there are clinical and radiological features suggestive of NPHP and age of end-stage renal failure is less than 5 years of age. If no mutations are found additional NPHP genes should be screened depending on phenotype and a differential diagnosis of MCKD, ARPKD and BBS should be considered.
Infantile NPHP
Early onset ESRF (less than 5 years of age)
Possible antenatal presentation with fetal oliguria and oligohydramnios10
Renal USS – normal sized or enlarged kidneys
Renal biopsy – interstitial fibrosis, tubular atrophy, absence of tubular basement membrane irregularity, renal cortical microcysts
Associated extrarenal features peculiar to infantile NPHP include hypertension, situs inversus, ventricular septal defect.
NPHP
Median onset of ESRF 12 years (may be beyond 25 years)9
Polyuria and polydipsia (and salt wasting) in early childhood (4–6 years of age)
Urinary concentration defect (<400 mosm/kg in early morning urine) that is not responsive to desmopressin
Growth retardation (secondary to salt wasting, dehydration and renal insufficiency)
Absence of (or minimal) haematuria and proteinuria
Renal USS – renal cortical hyperechogenicity, loss of corticomedullary differentiation, corticomedullary cysts11
Renal biopsy – microscopy typically shows interstitial nephritis, tubular atrophy and tubular dilatations. Typically there is both thickening and attenuation of the tubular basement membranes.
The clinical diagnosis of NPHP may be made (or looked for) following detection of an associated extrarenal disorder (see below and Table 2).
Table 2. Extra renal manifestations associated with NPHP.
| Eye/retinal disease |
| Isolated oculomotor apraxia |
| Retinitis pigmentosa |
| Coloboma |
| Nystagmus |
| Ptosis |
| Neurological disease |
| Learning difficulties |
| Cerebellar ataxia with vermis hypoplasia |
| Hypopituitarism |
| Encephalocoele |
| Liver disease |
| Elevation of hepatic enzymes |
| Fibrosis, biliary duct proliferation |
| Skeletal |
| Phalangeal cone-shaped epiphyses |
| Short ribs |
| Postaxial polydactyly |
| Skeletal dysplasia |
| Others |
| Situs inversus |
| Cardiac malformations |
| Bronchiectasis |
Nephronophthisis and disease associations
Many disorders have been described in which NPHP is a clinical feature. Such multisystem features and pleiotropy are typical of ‘ciliopathies' such as NPHP. Extrarenal manifestations are seen in 10–20% of cases of NPHP.12
Senior–Loken syndrome
Here retinal dysplasia and degeneration (also known as tapetoretinal degeneration or retinitis pigmentosa) may lead to early and severe visual loss (within 2 years of age), resembling Leber's congenital amaurosis (LCA). Later onset forms present initially with night blindness, which progresses to visual loss by the age of 10 years. Diagnosis is made by performing an electroretinogram, which may show abnormalities before the physical signs of retinitis pigmentosa and visual loss. Molecular mechanisms of blindness are secondary to photoreceptor cell defects (reviewed in reference13).
Joubert syndrome and related disorders
Joubert syndrome and related disorders (JSRD) are characterized by cerebellar vermis hypoplasia and brainstem abnormalities.5 Brain imaging (MRI) reveals a characteristic appearance of the brain stem known as the ‘molar tooth sign'. Typically an affected child will have an irregular breathing pattern in the newborn period and often abnormal eye movements. During infancy hypotonia develops, with ataxia developing late in childhood. Other conditions associated with JSRD include CNS anomalies, ocular coloboma, retinal dystrophy, skeletal defects such as polydactyly, hepatic fibrosis and cystic dysplastic kidneys or NPHP.
Oculomotor apraxia
This is characterized by abnormal eye movements, which include nystagmus and difficulty with saccades (smooth visual pursuits). The transient inability to perform horizontal gaze eye movements in the first years of life is referred to as oculomotor apraxia (OMA) type Cogan and is associated with NPHP gene mutations.14 Indeed, OMA may be a mild form of JSRD, as cerebellar vermis aplasia has been described in this condition.15
Skeletal defects
A variety of associated skeletal defects have been reported, the most frequent are cone-shaped epiphyses.16, 17 Scoliosis due to poor muscle tone (as part of a JSRD syndrome) and polydactyly (postaxial, most commonly) may also occur.
Cardiac defects
Situs inversus and other structural heart defects (cardiac ventricular sepal defect) have been reported in association with infantile NPHP.18, 19
Other rare associations
Other syndromes that include NPHP have been described. These include Ellis van Creveld syndrome,20 RHYNS (retinitis pigmentosa, hypopituitarism and skeletal dysplasia),21 Alstrom syndrome, COACH syndrome, Jeune syndrome and Arima syndrome (Table 1).
Meckel–Gruber like syndrome
The association of occipital encephalocoele, polydactyly and ductal proliferation in the portal area of the liver and cystic kidney dysplasia is known as Meckel–Gruber syndrome (MKS). Recently, mutations in some of the genes implicated in NPHP/JSRD have been found in patients with MKS.18, 22, 23, 24 This broadens the phenotypic spectrum of diseases associated with NPHP gene defects and implies a common pathogenesis.
Diagnostic approaches
Initial evaluation
Diagnosis relies on a clinical suspicion of the disorder. NPHP should initially be investigated non-invasively.
Key features would include:
History
polyuria and polydipsia, enuresis.
Complications of renal insufficiency/renal failure such as nausea, vomiting, itch, fatigue (anaemia), growth retardation.
Family history of renal disease (autosomal recessive pattern, consanguineous families)
Examination
Blood pressure
Extrarenal manifestations such as retinal pigmentation, abnormal eye movements and polydactyly.
Investigations
Urine dipstick (minimal proteinuria (<0.5 g/l) and minimal haematuria is typical).
Early morning urine to assess urinary concentration.
USS of abdomen and kidneys to assess renal size, to look for corticomedullary cysts, corticomedullary differentiation, to exclude renal tract dilatation and to examine for liver fibrosis/splenomegaly.
MRI scan and full neurological evaluation to assess cerebellar function if neurological symptoms.
A baseline ophthalmological examination is essential to look for minor degrees of coloboma, retinopathy, OMA. Visual evoked potential studies may be performed in newborn children. ERG studies may be performed from 8 months of age.
Blood tests: renal function (urea, creatinine), liver function (albumin, transaminases, bilirubin), full blood count (to look for renal anaemia) and clotting studies (prothrombin time as a marker of liver function and before renal biopsy, if necessary). If renal failure is advanced, screening for renal osteodystrophy, hyperparathyroidism and metabolic acidosis should be performed.
Genetic testing
Following appropriate genetic counselling, homozygous or heterozygous NPHP1 deletion (found in around 25% of cases) can be screened easily by PCR. Other NPHP genes may be tested by direct sequencing (see http://www.orpha.net for a list of laboratories). A renal biopsy should not be necessary if a molecular genetic diagnosis can be made. If a molecular diagnosis is not available, a renal biopsy may be required to confirm or exclude NPHP (Figure 2).
ESRF and disease management
Preparation for ESRF (renal replacement therapy) and consideration for renal transplantation should be undertaken during subsequent reviews of the patient, once a diagnosis has been made. NPHP does not recur in transplanted kidneys. Living-related kidney donation from unaffected family members, including heterozygous carriers (eg parents), is possible following clinical evaluation. Referral to the Joubert Syndrome Foundation (http://www.joubertsyndrome.org/) and other support organizations for families of children with disabilities (eg http://www.cafamily.org.uk/services.html or http://www.orpha.net) may be appropriate.
Differential diagnosis of NPHP
NPHP should not be confused with autosomal dominant polycystic kidney disease (ADPKD) which is characterized by bilateral, multiple renal cysts resulting in kidney enlargement over time, with extrarenal manifestations which include simple liver cysts, which arise from the biliary epithelium.
NPHP should be distinguished from medullary cystic kidney disease (MCKD), which shares pathological appearances at the macroscopic and microscopic level. However, unlike NPHP, MCKD is inherited in an autosomal dominant pattern, and the age of ESRF is usually later. Two different variants of MCKD are known, MCKD1 (gene remains unidentified) and MCKD2 (secondary to UMOD mutations), with a median onset of ESRD at 62 and 32 years,25 respectively. In contrast to NPHP, the only extra-renal manifestation of MCKD is the occurrence of hyperuricaemia and gout.25
Given the antenatal/early childhood onset of renal disease in infantile NPHP, care must be taken to exclude autosomal recessive polycystic kidney disease (ARPKD; Figure 2). Like NPHP, ARPKD may present at a wide age distribution, from antenatally to adulthood. Antenatal ultrasound scanning may reveal markedly enlarged kidneys with increased echogencity. Kidney microcysts and fusiform dilation of collecting ducts are typical of ARPKD. Liver involvement is always present in ARPKD and may be the predominant clinical feature, with dilated intrahepatic bile ducts, liver fibrosis and portal hypertension. The gene defect is in the PKHD1 gene, encoding its protein product fibrocystin (or polyductin).26
Finally, Bardet–Biedl syndrome (BBS) must be considered in the differential diagnosis of NPHP (Figure 2). BBS is another ciliopathy affecting multiple organ systems.27 Clinical features may include obesity, learning difficulties, genitourinary tract malformations and limb deformities.28 Renal lesions may include renal cysts, dysplasia, concentrating defects and progressive renal failure.28 Histologically, cystic dilatation of the renal collecting ducts have been described,29 reminiscent of infantile NPHP.
Molecular and genetic basis of NPHP
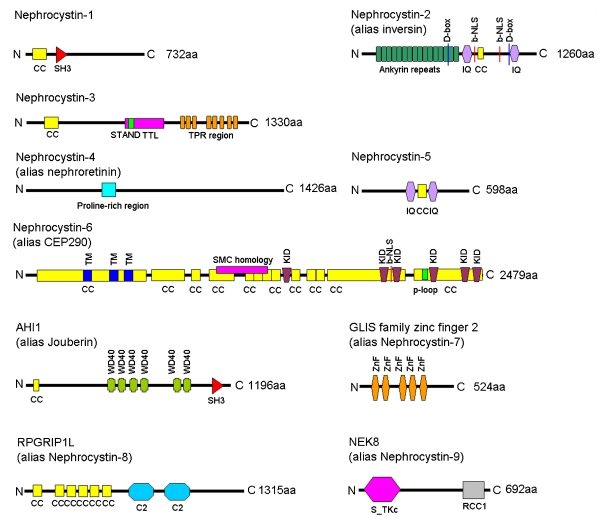
There are a growing number of genes implicated in NPHP. These will be briefly reviewed in terms of their phenotype, frequency and most common disease associations. NPHP is largely inherited as an autosomal recessive disease with homozygous single gene mutations/deletions or compound heterozygous mutations occurring in a single NPHP gene. This usually allows a molecular diagnosis and accurate genetic counselling to be performed. However oligogenicity, where allelic variants at multiple loci contribute to disease, has been documented for NPHP.30 Likewise, additional NPHP gene mutations may modulate the phenotype in an epistatic way.31 Thus a wide spectrum of clinical variants with any mutant gene(s) is possible (Table 3). The encoded NPHP proteins, called nephrocystins, typically posses multiple domains (Figure 3).
Table 3. Genetics defects underlying NPHP, associated features and other clinical phenotypes.
| Gene (protein) | Chromosome | NPHP type | Clinical features associated with NPHP | Other clinical phenotypes |
|---|---|---|---|---|
| NPHP1 (nephrocystin-1) | 2q13 | NPHP | Mild JS, mild RP, Cogan | JS |
| NPHP2/INVS (inversin) | 9q31 | Infantile NPHP | RP, liver fibrosis, situs inversus, hypertension, VSD | |
| NPHP3 (nephrocystin-3) | 3q22 | NPHP, Infantile NPHP | Liver fibrosis, RP, situs inversus | MKS |
| NPHP4 (nephrocystin-4 or nephroretinin) | 1p36 | NPHP | Cogan, RP | |
| NPHP5/IQCB1 (nephrocystin-5) | 3q21 | NPHP | Severe RP | |
| NPHP6/CEP290 (nephrocystin-6/CEP290) | 12q21 | NPHP | JS, severe RP | Isolated RP, JS, MKS, BBS |
| NPHP7/GLIS2 (GLIS2) | 16p | NPHP | ||
| NPHP8/RPGRIP1L (RPGRIP1L) | 16q | NPHP | JS | JS, MKS |
| NPHP9/NEK8 (NEK8) | 17q11 | NPHP, Infantile NPHP | ||
| AHI1 (Jouberin/AHI1) | 6q23 | NPHP | JS, RP | JS |
JS, Joubert syndrome type B; RP, retinitis pigmentosa; Cogan, oculomotor apraxia type Cogan; MKS, Meckel–Gruber syndrome; VSD, ventricular septal defect.
Figure 3.
Nephrocystin proteins and their protein domains. Domain structure of the nephrocystin proteins. Nephrocystin proteins contain a diverse variety of protein domains and no common pattern can be identified. Nephrocystin-1, encoded by NPHP1, is a 732 amino acid (aa) protein, which possesses an N-terminal coiled-coil domain (CC) and a Src-homology 3 domain (SH3). Nephrocystin-2 (alias inversin), encoded by NPHP2/INVS, is a 1260 aa protein with 16 tandem ankyrin repeats, two IQ calmodulin binding domains (IQ). There are two destruction-box (D-box) regions (the first of which is Apc2 binding) and a bipartite nuclear localization signal (b-NLS) and a putative coiled-coil (CC) domain. Nephrocystin-3, encoded by NPHP3, is a 1330 aa protein, with a coiled-coil domain (CC), a tetratricopeptide-repeat domain (TPR) and a tubulin tyrosine ligase domain (TTL). Within the TTL a STAND (signal transduction ATPases with numerous domains) domain, which may be found in P-loop NTPases, is located. Nephrocystin-4 (alias nephroretinin), encoded by NPHP4 is a 1426 aa protein, which lacks any known domains. There is a central proline rich region. Nephrocystin-5, encoded by NPHP5/IQCB1 is a 598 aa protein. This protein possesses two IQ calmodulin binding sites, which surround a putative coiled-coil (CC) domain. Nephrocystin-6 (alias CEP290) is encoded by NPHP6/CEP290. This is a 2479 aa protein with multiple domains which include 13 coiled-coil (CC) domains; 3 tropomyosin homology domains (TM); 6 RepA/Rep+ protein KID motifs (KID); a bipartite nuclear localization signal (b-NLS); a ATP/GTP-binding site motif A (p-loop). The extent of homology with Structural Maintenance of Chromosomes proteins (SMC) is also indicated. AHI1 (alias Jouberin) is encoded by AHI1 and is an 1196 aa protein, which contains a Src-homology 3 domain (SH3), 6 WD40 domains (WD40) and an N-terminal coiled-coil domain. GLIS family zinc finger 2 (alias nephrocystin-7) is a 524 aa protein encoded by GLIS2. It contains 5 zinc finger domains (ZnF). RPGRIP1L (alias nephrocystin-8) is a 1315 aa protein encoded by RPGRIP1L. Protein domains include 6 coiled-coil (CC) domains and two protein kinase C conserved region 2 (C2) domains. The C-terminal C2 domain mediates the interaction with nephrocystin-4. NEK8 (alias nephrocystin-9) is a 692 aa protein with a serine/threonine protein kinases, catalytic domain (S-TKc) and a regulator of chromosome condensation (RCC1) domain, which is highly conserved throughout evolution.
NPHP1 and nephrocystin-1
NPHP1 was the first NPHP gene identified, using positional cloning strategies in consanguineous families.32, 33 Homozygous deletions of ∼250 kb DNA in the region 2q13 are the most frequent genetic abnormality found.34 Other mutations include compound heterozygosity for the NPHP1 gene deletion combined with a single point mutation in the NPHP1 gene. NPHP1 mutations account for about 25% of cases of NPHP. NPHP1 mutations may be associated with congenital OMA type Cogan14 and Senior–Loken syndrome35 and also give rise to JSRD phenotypes.31, 36
NPHP1 encodes a protein product named nephrocystin-1. Nephrocystin-1 has been localized to the primary renal cilium19 and to epithelia cell adherens junctions.37, 38 More recently, the primary cilial localization has been refined to the transition zone (at the ciliary base) in renal and respiratory epithelia and to the connecting cilia in photoreceptor cells.39 Targeting of nephrocystin-1 to the transition zone of the cilia is dependent on casein kinase 2 phosphorylation and an interaction with PACS-1.40 Nephrocystin-1 also interacts with other nephrocystins (Nephrocystin-2, -3, -4 and Jouberin16, 41, 42, 43, 44) and there is evidence that this complex of proteins may function in multiple intracellular locations including the cilium, cell–cell adherens junctions and at focal adhesions.19, 37, 38, 44, 45 Within the human kidney nephrocystin-1 is expressed in renal collecting ducts.44
INVS/NPHP2 and inversin
Mutations in INVS/NPHP2 give rise to infantile NPHP.19 These mutations are rare and account for <1% of all cases of NPHP worldwide. The gene encodes the protein named inversin, which has a dynamic distribution during cell cycle46 and is expressed in renal cilia.19, 46, 47 INVS mutations may cause situs inversus in affected patients, and knockout animals mimic the human disease, with large cystic kidneys at an early age, situs inversus and hepatobiliary malformations.48 Retinitis pigmentosa is an uncommon but reported association with INVS mutations.49 Inversin seems to play a crucial role in Wnt signalling, acting as a switch between canonical and non-canonical Wnt signalling pathways50, 51 and is required for convergent extension movements.50 This suggests that inversin plays a role in the developing nephron and in maintenance of the tubular architecture. This coordinated ability of epithelial cells to divide and reorganize themselves to form and maintain tubular structures relies on planar cell polarity (PCP) signalling. PCP signalling is mediated via proteins associated with the primary cilia/basal body complex, such as inversin50 and its disruption may underlie the pathophysiology of cyst development.51
NPHP3 and nephrocystin-3
Mutations in NPHP3 can produce diverse phenotypes. Mutations were originally identified in a large Venezuelan kindred who exhibited NPHP.16 Mutations in NPHP3 were associated with hepatic fibrosis and retinal degeneration in some affected individuals.16 Recently the phenotype of NPHP3 mutations has been expanded to include Meckel–Gruber like syndrome.18
NPHP3 encodes nephrocystin-3, which interacts with nephrocystin-116 and inversin,18 and can inhibit canonical Wnt signalling. A mouse model of NPHP type 3, named pcy, displays cystic kidney disease which responds to treatment with the aquaretic agents/vasopressin-2-receptor antagonists.52
NPHP4 and nephrocystin-4 (alias nephroretinin)
NPHP4 encodes nephrocystin-4 (alias nephroretinin), a highly conserved protein which interacts with nephrocystin-1.42 Nephrocytsin-4 complexes with α-tubulin and localizes to the primary cilium and basal bodies.41 NPHP4 mutations may cause isolated NPHP, NPHP with RP and NPHP with OMA.53 Recently, nephrocystin-4 has been reported to interact with RPGRIP1L.24, 54
NPHP5 and nephrocystin-5
The NPHP5/IQCB1 gene encodes nephrocystin-5. This protein contains two IQ calmodulin binding sites, which surround a coiled-coil domain. Similar to inversin, nephrocystin-5 interacts directly with calmodulin via its IQ domains, with which it colocalizes to the primary cilium, and forms a complex with RPGR.55 The clinical phenotype of NPHP5 mutations is always associated with severe retinal degeneration (early onset Senior–Loken syndrome).
NPHP6/CEP290 and nephrocystin-6
The NPHP6 (alias CEP290) gene encodes the nephrocystin-6 protein. Mutations in NPHP6 account for a growing spectrum of clinical phenotypes which include isolated NPHP, Senior–Loken syndrome, JSRD,56, 57 MKS22, 23 and BBS.58 Mutations in NPHP6 have also been described in 21% patients with isolated LCA, making this the most common gene defect for isolated LCA.59 A mouse model, named rd16 has an in-frame deletion in Nphp6/Cep290 and mimics this phenotype, with early onset retinal degeneration, but no kidney or brain disease. Nephrocystin-6 directly interacts with and activates the cAMP related transcription factor, CREB2 (alias ATF4).56 Interestingly, single heterozygous mutations in NPHP6 have been described in individuals with NPHP who have NPHP1 homozygous deletions.31 Similarly, a heterozygous nonsense mutation in NPHP6 was described together with a heterozygous NPHP4 missense mutation in an individual affected with Senior–Loken syndrome.57 This tendency towards digenic and oligogenicity has recently been reported for other NPHP genes.30, 60
NPHP7/GLIS2 and GLIS2
The NPHP7/GLIS2 gene encodes the Kruppel-like zinc-finger transcription factor GLIS2 that localizes to both the primary cilia and the nucleus.61 Mutations were reported in a consanguineous Oji-Cree Canadian family with affected members having isolated NPHP and early onset renal failure (by 8 years of age) but remains a rare genetic cause of NPHP.61 A mouse model of targeted Glis2 disruption within the kidney reveals increased rates of apoptosis, with tubular atrophy and fibrosis.
NPHP8/RPGRIP1L and RPGRIP1L
The RPGRIP1L gene encodes a protein named retinitis pigmentosa GTPase regulator interacting protein 1-like protein (RPGRIP1L). Mutations were initially reported in fetuses affected with MKS and patients with JSRD.24, 62 Additional features in some patients included scoliosis, polydactyly, pituitary agenesis and partial growth hormone deficiency, reminiscent of RHYNS syndrome.62 Regarding RPGRIP1L mutations, some phenotype–genotype correlations can be drawn as homozygous truncating mutations seem to cause MKS24, 62 whereas a heterozygous truncating mutation or a homozygous missense mutation causes JSRD. RPGRIP1L is a centrosomal protein, which interacts with nephrocystin-4. JSRD causing mutations in RPGRIP1L confer loss off interaction with nephrocystin-4.24
A mouse model Ftm−/− (Fantom or fused-toe mouse) represents inactivation of the mouse ortholog Rpgrip1l (Ftm) and recapitulates the cerebral, renal and hepatic defects of JSRD and MKS.
NPHP9/NEK8 and NEK8
The NEK8 gene encodes the NEK8 protein (never in mitosis A-related kinase 8). Mutations have been described in two families with NPHP and one consanguineous family with infantile NPHP. In one NPHP family with a homozygous NPHP5 mutation, which accounts for the disease phenotype, a single heterozygous NEK8 mutation was found.60 These findings demonstrate firstly, the rarity of NEK8 mutations and secondly, that NEK8 mutations may contribute to oligogenicity in patients with NPHP. The jck mouse model of cystic kidney disease contains a missense mutation (G448V) in Nek8. Nek8 and polycystin-2 form a protein complex together, which adds weight to the argument that there are common mechanisms underlying NPHP and ADPKD.63, 64
AHI1 and AHI1/Jouberin protein
The AHI1 (Abelson helper integration site 1) gene encodes the AHI1 protein, which is also known as Jouberin. Mutations in AHI1 were initially described in individuals with a JSRD phenotype, with no renal disease.65, 66 Subsequently, AHI1 mutations were found in individuals with NPHP67 and with retinal degeneration.68 Jouberin is localized to adherens junctions, basal bodies and primary cilia.69 Jouberin interacts with nephrocystin-1, and has been localized to the renal collecting duct.69
Other NPHP genes
NPHP1 gene mutations account for around 25% of all cases of NPHP. The remaining nine genes are each found in 0.05–3% of cases, and collectively probably only account for another 25% of cases of NPHP, meaning that many cases remain ‘unsolved'. For JSRD, at least two additional loci have been reported. These are JBTS1 on chromosome 9q3470 and JBTS2 (CORS2) on chromosome 11 (a large pericentromeric region).71 Patients linked to the JBTS2 locus often have renal disease as part of their disease spectrum. Very recently, mutations in ARL13B, which encodes a cilial protein, were found in patients with classical JS, with no renal phenotype.72
The role of the primary cilia in NPHP
The identification of genetic causes of NPHP has highlighted the paradigm, that all protein products of cystic kidney diseases are expressed in the primary renal cilium/basal body complex.73 The primary cilium is present on nearly every cell in the human body and is a cell surface projection which acts as an ‘antenna'. This organelle extends from the basal body and consists of an axoneme comprising nine microtubular doublets. Assembly of the axoneme occurs via a process called IFT where proteins are moved up and down the cilium.73 Nephrocystins are located within this cilial subcellular domain, where they form complexes with themselves and other related proteins, probably to facilitate signalling cascades. Primary cilia are thought to sense tubular luminal flow (of urine) and regulate calcium entry (mediated by polycystin-2 channels).74 Nephrocystins are expressed in the connecting cilium of the photoreceptor cell of the retina and defects here correlate with retinal defects and degeneration, often associated with NPHP gene mutations. Related syndromes such as Jeune syndrome and Ellis van Creveld syndrome (EVC) have held true to the cilial paradigm. Jeune syndrome is secondary to mutations in the IFT protein IFT8075 and EVC (together with EVC2) mutations underlie EVC, and encodes a cilial/basal body protein.76
Management
Clinical work up (see ‘diagnostic approaches' section for details of initial evaluation)
Surveillance and management
Given that the renal disease NPHP is often being managed in the context of extra-renal manifestations, ongoing surveillance of affected patients by appropriate specialists is important.
Regular evaluations
Patients with NPHP will invariably progress to end-stage renal failure. Management in a ‘low clearance' setting is appropriate to allow time for consideration of renal replacement therapies. USS scans may detect renal cystic changes as the disease progresses. Growth, endocrine and sexual maturation and neurological evaluations should be regularly performed. Retinal disease may become progressive. Annual eye examinations commencing at the time of diagnosis is recommended.5 Liver function tests should be performed regularly and liver ultrasound scan should be performed if suspicion of liver disease.
Genetic testing for NPHP
NPHP is a genetically heterogeneous disorder, however testing for the most common gene defect, a homozygous deletion of NPHP1 (Figure 2), is readily available (see http://www.ukgtn.nhs.uk/gtn/Home; http://www.orpha.net and http://www.genetests.org/). Direct sequencing of other NPHP genes may also be performed (see http://autozygosity.org/diagnostic; http://www.renalgenes.org/ and http://www.orpha.net). Technologies are however changing rapidly and given that the genomic regions covered by all known NPHP genes is less than 1 mb (Table 4) a gene capture service followed by use of high throughput sequencing platforms may allow an efficient way of screening patients with NPHP in the near future. Indeed, with the recent descriptions of oligogenicity30 and epistasis31 in NPHP, testing of all NPHP associated genes may be important to understand this complex disorder.
Table 4. Coding exon numbers and genomic sequence length in NPHP genes.
| Gene | Coding exons | Genomic DNA (bp) |
|---|---|---|
| NPHP1 | 20 | 82 691 |
| INVS/NPHP2 | 16 | 201 916 |
| NPHP3 | 27 | 41 823 |
| NPHP4 | 29 | 129 662 |
| NPHP5 | 13 | 65 009 |
| NPHP6/CEP290 | 53 | 93 204 |
| NPHP7/GLIS2 | 6 | 7383 |
| NPHP8/RPGRIP1L | 26 | 103 954 |
| NPHP9/NEK8 | 15 | 13 953 |
| AHI1 | 23 | 109 962 |
| Total | 228 | 849 557 |
Genetic counselling
Genetic testing should not be performed before appropriate consent and genetic counselling. NPHP is inherited in an autosomal recessive manner, however in some affected individuals more than one associated gene may contribute to disease.30 Such oligogenicity has also been reported in BBS.77 In general, NPHP is a moderately severe disorder with major impacts on renal function and other aspects of health and development. The variable severity of the disorder in different families and even between individuals within families makes predicting outcome difficult.
Prenatal diagnosis
For families with a genetic diagnosis of NPHP, prenatal testing is possible. Prenatal imaging may reveal cystic kidney disease and other abnormalities (such as structural CNS lesions and polydactyly) in at-risk pregnancies. INVS/NPHP2 mutations typically produce a prenatal cystic phenotype, as may the genes which have been reported to give a MKS-like phenotype (Table 3).
Treatment and care
At present there are no proven treatments for NPHP. Treatment must centre on the progressive renal failure, which leads to ESRF and the need for dialysis and transplantation. Potential treatments, targeted towards the collecting duct may be available for future use. These include vasopressin V2 receptor antagonists, which may alter cystogenesis and progression of disease. The pcy mouse model of NPHP type 3 responded to treatment with OPC31260.52 Evidence is also growing for use of rapamycin, an mTOR inhibitor, for reducing renal cystogenesis.78, 79
Conclusion
During the past decade significant insight has been made in the molecular genetics of NPHP. This disease has moved from being a pathological description to an inherited ciliopathy, whereby the most common form may be readily detected by gene testing, without the need for a renal biopsy. Additional genes will no doubt be found, and high throughput technologies show promise for providing a screen of all currently known genes implicated in ciliopathies. The major challenge remains to understand the biological function of nephrocystin proteins, the molecular mechanisms that lead to renal failure and the potential treatments, which may prevent or reverse these changes. In practical terms, NPHP must be considered among the differential diagnosis of any cause of renal failure of unknown origin. The recognition that NPHP is part of a ciliopathy, with a wide clinical spectrum of disease will allow earlier diagnosis to be made, allowing for time for genetic counselling, appropriate genetic testing and improved treatment planning for ESRF.
Acknowledgments
RJS is funded by a Mason Medical Research Fellowship. JAS is funded by GlaxoSmithKline (Clinician Scientist Award).
References
- Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- Zollinger HU, Mihatsch MJ, Edefonti A, Gaboardi F, Imbasciati E, Lennert T. Nephronophthisis (medullary cystic disease of the kidney). A study using electron microscopy, immunofluorescence, and a review of the morphological findings. Helv Paediatr Acta. 1980;35:509–530. [PubMed] [Google Scholar]
- Krishnan R, Eley L, Sayer JA. Urinary concentration defects and mechanisms underlying nephronophthisis. Kidney Blood Press Res. 2008;31:152–162. doi: 10.1159/000129648. [DOI] [PubMed] [Google Scholar]
- Gagnadoux MF, Bacri JL, Broyer M, Habib R. Infantile chronic tubulo-interstitial nephritis with cortical microcysts: variant of nephronophthisis or new disease entity. Pediatr Nephrol. 1989;3:50–55. doi: 10.1007/BF00859626. [DOI] [PubMed] [Google Scholar]
- Parisi MA, Doherty D, Chance PF, Glass IA. Joubert syndrome (and related disorders) (OMIM 213300) Eur J Hum Genet. 2007;15:511–521. doi: 10.1038/sj.ejhg.5201648. [DOI] [PubMed] [Google Scholar]
- Waldherr R, Lennert T, Weber HP, Fodisch HJ, Scharer K. The nephronophthisis complex. A clinicopathologic study in children. Virchows Arch A Pathol Anat Histol. 1982;394:235–254. doi: 10.1007/BF00430668. [DOI] [PubMed] [Google Scholar]
- Potter DE, Holliday MA, Piel CF, Feduska NJ, Belzer FO, Salvatierra O., Jr Treatment of end-stage renal disease in children: a 15-year experience. Kidney Int. 1980;18:103–109. doi: 10.1038/ki.1980.115. [DOI] [PubMed] [Google Scholar]
- Ala-Mello S, Sankila EM, Koskimies O, de la Chapelle A, Kääriäinen H. Molecular studies in Finnish patients with familial juvenile nephronophthisis exclude a founder effect and support a common mutation causing mechanism. J Med Genet. 1998;35:279–283. doi: 10.1136/jmg.35.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollee G, Fakhouri F, Karras A, et al. Nephronophthisis related to homozygous NPHP1 gene deletion as a cause of chronic renal failure in adults. Nephrol Dial Transplant. 2006;21:2660–2663. doi: 10.1093/ndt/gfl348. [DOI] [PubMed] [Google Scholar]
- Haider NB, Carmi R, Shalev H, Sheffield VC, Landau D. A Bedouin kindred with infantile nephronophthisis demonstrates linkage to chromosome 9 by homozygosity mapping. Am J Hum Genet. 1998;63:1404–1410. doi: 10.1086/302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera A, Rivera M, Gallego N, Nogueira J, Ortuno J. Sonographic appearance of the juvenile nephronophthisis-cystic renal medulla complex. Nephrol Dial Transplant. 1997;12:625–626. doi: 10.1093/ndt/12.3.625. [DOI] [PubMed] [Google Scholar]
- Salomon R, Saunier S, Niaudet P.Nephronophthisis Pediatr Nephrol 2008[Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Adams NA, Awadein A, Toma HS. The retinal ciliopathies. Ophthalmic Genet. 2007;28:113–125. doi: 10.1080/13816810701537424. [DOI] [PubMed] [Google Scholar]
- Betz R, Rensing C, Otto E, et al. Children with ocular motor apraxia type Cogan carry deletions in the gene (NPHP1) for juvenile nephronophthisis. J Pediatr. 2000;136:828–831. [PubMed] [Google Scholar]
- Harris CM, Hodgkins PR, Kriss A, et al. Familial congenital saccade initiation failure and isolated cerebellar vermis hypoplasia. Dev Med Child Neurol. 1998;40:775–779. doi: 10.1111/j.1469-8749.1998.tb12347.x. [DOI] [PubMed] [Google Scholar]
- Olbrich H, Fliegauf M, Hoefele J, et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet. 2003;34:455–459. doi: 10.1038/ng1216. [DOI] [PubMed] [Google Scholar]
- Ellis DS, Heckenlively JR, Martin CL, Lachman RS, Sakati NA, Rimoin DL. Leber's congenital amaurosis associated with familial juvenile nephronophthisis and cone-shaped epiphyses of the hands (the Saldino-Mainzer syndrome) Am J Ophthalmol. 1984;97:233–239. doi: 10.1016/s0002-9394(14)76095-7. [DOI] [PubMed] [Google Scholar]
- Bergmann C, Fliegauf M, Bruchle NO, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudgil A, Bagga A, Kamil ES, et al. Nephronophthisis associated with Ellis-van Creveld syndrome. Pediatr Nephrol. 1998;12:20–22. doi: 10.1007/s004670050395. [DOI] [PubMed] [Google Scholar]
- Di Rocco M, Picco P, Arslanian A, et al. Retinitis pigmentosa, hypopituitarism, nephronophthisis, and mild skeletal dysplasia (RHYNS): a new syndrome. Am J Med Genet. 1997;73:1–4. doi: 10.1002/(sici)1096-8628(19971128)73:1<1::aid-ajmg1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Frank V, den Hollander AI, Bruchle NO, et al. Mutations of the CEP290 gene encoding a centrosomal protein cause Meckel-Gruber syndrome. Hum Mutat. 2008;29:45–52. doi: 10.1002/humu.20614. [DOI] [PubMed] [Google Scholar]
- Baala L, Audollent S, Martinovic J, et al. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007;81:170–179. doi: 10.1086/519494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- Scolari F, Ghiggeri GM, Amoroso A, Caridi GL, Aridon P. Genetic heterogeneity for autosomal dominant medullary cystic kidney disease (ADMCKD) J Am Soc Nephrol. 1998;9:393A. [Google Scholar]
- Ward CJ, Hogan MC, Rossetti S, et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- Adams M, Smith UM, Logan CV, Johnson CA. Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J Med Genet. 2008;45:257–267. doi: 10.1136/jmg.2007.054999. [DOI] [PubMed] [Google Scholar]
- Tobin JL, Beales PL. Bardet-Biedl syndrome: beyond the cilium. Pediatr Nephrol. 2007;22:926–936. doi: 10.1007/s00467-007-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RM, Dery P, Norady MB, Drummond KN. The renal lesion of the Laurence-Moon-Biedl syndrome. J Pediatr. 1975;87:206–209. doi: 10.1016/s0022-3476(75)80580-4. [DOI] [PubMed] [Google Scholar]
- Hoefele J, Wolf MT, O'Toole JF, et al. Evidence of oligogenic inheritance in nephronophthisis. J Am Soc Nephrol. 2007;18:2789–2795. doi: 10.1681/ASN.2007020243. [DOI] [PubMed] [Google Scholar]
- Tory K, Lacoste T, Burglen L, et al. High NPHP1 and NPHP6 mutation rate in patients with Joubert syndrome and nephronophthisis: potential epistatic effect of NPHP6 and AHI1 mutations in patients with NPHP1 mutations. J Am Soc Nephrol. 2007;18:1566–1575. doi: 10.1681/ASN.2006101164. [DOI] [PubMed] [Google Scholar]
- Antignac C, Arduy CH, Beckmann JS, et al. A gene for familial juvenile nephronophthisis (recessive medullary cystic kidney disease) maps to chromosome 2p. Nat Genet. 1993;3:342–345. doi: 10.1038/ng0493-342. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Otto E, Rensing C, et al. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet. 1997;17:149–153. doi: 10.1038/ng1097-149. [DOI] [PubMed] [Google Scholar]
- Konrad M, Saunier S, Calado J, Gubler MC, Broyer M, Antignac C. Familial juvenile nephronophthisis. J Mol Med. 1998;76:310–316. doi: 10.1007/s001090050222. [DOI] [PubMed] [Google Scholar]
- Otto EA, Helou J, Allen SJ, et al. Mutation analysis in nephronophthisis using a combined approach of homozygosity mapping, CEL I endonuclease cleavage, and direct sequencing. Hum Mutat. 2008;29:418–426. doi: 10.1002/humu.20669. [DOI] [PubMed] [Google Scholar]
- Castori M, Valente EM, Donati MA, et al. NPHP1 gene deletion is a rare cause of Joubert syndrome related disorders. J Med Genet. 2005;42:e9. doi: 10.1136/jmg.2004.027375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JC, Dempsey PJ, Reddy S, Bouton AH, Coffey RJ, Hanks SK. Crk-associated substrate p130(Cas) interacts with nephrocystin and both proteins localize to cell-cell contacts of polarized epithelial cells. Exp Cell Res. 2000;256:168–178. doi: 10.1006/excr.2000.4822. [DOI] [PubMed] [Google Scholar]
- Benzing T, Gerke P, Hopker K, Hildebrandt F, Kim E, Walz G. Nephrocystin interacts with Pyk2, p130(Cas), and tensin and triggers phosphorylation of Pyk2. Proc Natl Acad Sci USA. 2001;98:9784–9789. doi: 10.1073/pnas.171269898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M, Horvath J, von Schnakenburg C, et al. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J Am Soc Nephrol. 2006;17:2424–2433. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- Schermer B, Hopker K, Omran H, et al. Phosphorylation by casein kinase 2 induces PACS-1 binding of nephrocystin and targeting to cilia. EMBO J. 2005;24:4415–4424. doi: 10.1038/sj.emboj.7600885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet G, Silbermann F, Delous M, Salomon R, Antignac C, Saunier S. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum Mol Genet. 2005;14:645–656. doi: 10.1093/hmg/ddi061. [DOI] [PubMed] [Google Scholar]
- Mollet G, Salomon R, Gribouval O, et al. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat Genet. 2002;32:300–305. doi: 10.1038/ng996. [DOI] [PubMed] [Google Scholar]
- Otto E, Hoefele J, Ruf R, et al. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet. 2002;71:1167–1171. doi: 10.1086/344395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley L, Moochhala SH, Simms R, Hildebrandt F, Sayer JA. Nephrocystin-1 interacts directly with Ack1 and is expressed in human collecting duct. Biochem Biophys Res Commun. 2008;371:877–882. doi: 10.1016/j.bbrc.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Watnick T, Germino G. From cilia to cyst. Nat Genet. 2003;34:355–356. doi: 10.1038/ng0803-355. [DOI] [PubMed] [Google Scholar]
- Morgan D, Eley L, Sayer J, et al. Expression analyses and interaction with the anaphase promoting complex protein Apc2 suggest a role for inversin in primary cilia and involvement in the cell cycle. Hum Mol Genet. 2002;11:3345–3350. doi: 10.1093/hmg/11.26.3345. [DOI] [PubMed] [Google Scholar]
- Eley L, Turnpenny L, Yates LM, et al. A perspective on inversin. Cell Biol Int. 2004;28:119–124. doi: 10.1016/j.cellbi.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Phillips CL, Miller KJ, Filson AJ, et al. Renal cysts of inv/inv mice resemble early infantile nephronophthisis. J Am Soc Nephrol. 2004;15:1744–1755. doi: 10.1097/01.asn.0000131520.07008.b3. [DOI] [PubMed] [Google Scholar]
- O'Toole JF, Otto E, Frishberg Y, F H. Retinitis pigmentosa and renal failure in a patient with mutations in inversin. J Am Soc Nephrol. 2004;15:215A. doi: 10.1093/ndt/gfl088. [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germino GG. Linking cilia to Wnts. Nat Genet. 2005;37:455–457. doi: 10.1038/ng0505-455. [DOI] [PubMed] [Google Scholar]
- Gattone VH, II, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- Hoefele J, Sudbrak R, Reinhardt R, et al. Mutational analysis of the NPHP4 gene in 250 patients with nephronophthisis. Hum Mutat. 2005;25:411. doi: 10.1002/humu.9326. [DOI] [PubMed] [Google Scholar]
- Arts HH, Doherty D, van Beersum SE, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–888. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- Otto EA, Loeys B, Khanna H, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005;37:282–288. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- Sayer JA, Otto EA, O'Toole JF, et al. A novel centrosomal protein, nephrocystin-6, is mutated in Joubert syndrome and activates transcription factor ATF4/CREB2. Nat Genet. 2006;38:674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- Helou J, Otto EA, Attanasio M, et al. Mutation analysis of NPHP6/CEP290 in patients with Joubert syndrome and Senior-Loken syndrome. J Med Genet. 2007;44:657–663. doi: 10.1136/jmg.2007.052027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch CC, Zaghloul NA, Davis EE, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet. 2008;40:443–448. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Koenekoop RK, Yzer S, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J Am Soc Nephrol. 2008;19:587–592. doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attanasio M, Uhlenhaut NH, Sousa VH, et al. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat Genet. 2007;39:1018–1024. doi: 10.1038/ng2072. [DOI] [PubMed] [Google Scholar]
- Wolf MT, Saunier S, O'Toole JF, et al. Mutational analysis of the RPGRIP1L gene in patients with Joubert syndrome and nephronophthisis. Kidney Int. 2007;72:1520–1526. doi: 10.1038/sj.ki.5002630. [DOI] [PubMed] [Google Scholar]
- Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol. 2008;19:469–476. doi: 10.1681/ASN.2006090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli TA, Gareski TC, Dackowski WR, et al. Pkd1 and Nek8 mutations affect cell-cell adhesion and cilia in cysts formed in kidney organ cultures. Am J Physiol Renal Physiol. 2008;294:F73–F83. doi: 10.1152/ajprenal.00362.2007. [DOI] [PubMed] [Google Scholar]
- Ferland RJ, Eyaid W, Collura RV, et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet. 2004;36:1008–1013. doi: 10.1038/ng1419. [DOI] [PubMed] [Google Scholar]
- Dixon-Salazar T, Silhavy JL, Marsh SE, et al. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet. 2004;75:979–987. doi: 10.1086/425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsch B, Sayer JA, Attanasio M, et al. Identification of the first AHI1 gene mutations in nephronophthisis-associated Joubert syndrome. Pediatr Nephrol. 2006;21:32–35. doi: 10.1007/s00467-005-2054-y. [DOI] [PubMed] [Google Scholar]
- Parisi MA, Doherty D, Eckert ML, et al. AHI1 mutations cause both retinal dystrophy and renal cystic disease in Joubert syndrome. J Med Genet. 2006;43:334–339. doi: 10.1136/jmg.2005.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley L, Gabrielides C, Adams M, Johnson CA, Hildebrandt F, Sayer JA. Jouberin localizes to collecting ducts and interacts with nephrocystin-1. Kidney Int. 2008;74:1139–1149. doi: 10.1038/ki.2008.377. [DOI] [PubMed] [Google Scholar]
- Saar K, Al-Gazali L, Sztriha L, et al. Homozygosity mapping in families with Joubert syndrome identifies a locus on chromosome 9q34.3 and evidence for genetic heterogeneity. Am J Hum Genet. 1999;65:1666–1671. doi: 10.1086/302655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Salpietro DC, Brancati F, et al. Description, nomenclature, and mapping of a novel cerebello-renal syndrome with the molar tooth malformation. Am J Hum Genet. 2003;73:663–670. doi: 10.1086/378241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantagrel V, Silhavy JL, Bielas SL, et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83:170–179. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease. Nat Rev Genet. 2005;6:928–940. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Beales PL, Bland E, Tobin JL, et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–729. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Blair HJ, Rodriguez-Andres ME, et al. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134:2903–2912. doi: 10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- Badano JL, Kim JC, Hoskins BE, et al. Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet-Biedl patients with two mutations at a second BBS locus. Hum Mol Genet. 2003;12:1651–1659. doi: 10.1093/hmg/ddg188. [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin JL, Beales PL. Restoration of renal function in zebrafish models of ciliopathies. Pediatr Nephrol. 2008;23:2095–2099. doi: 10.1007/s00467-008-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]