Abstract
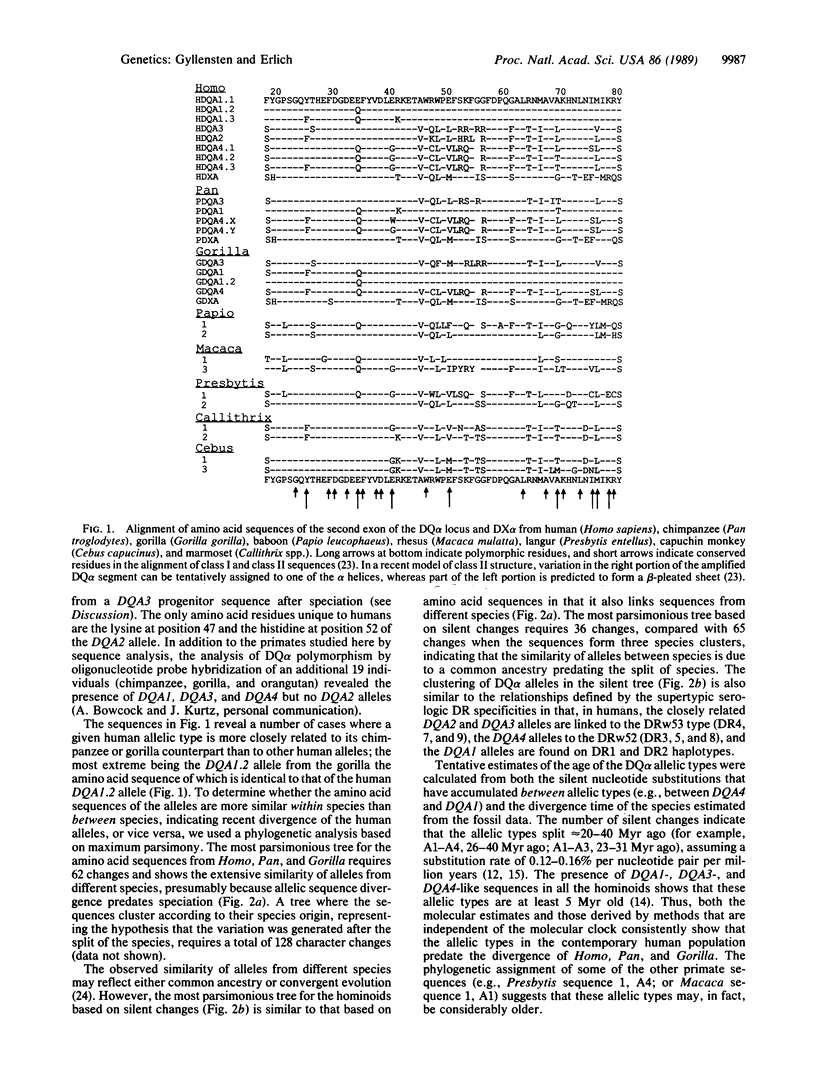
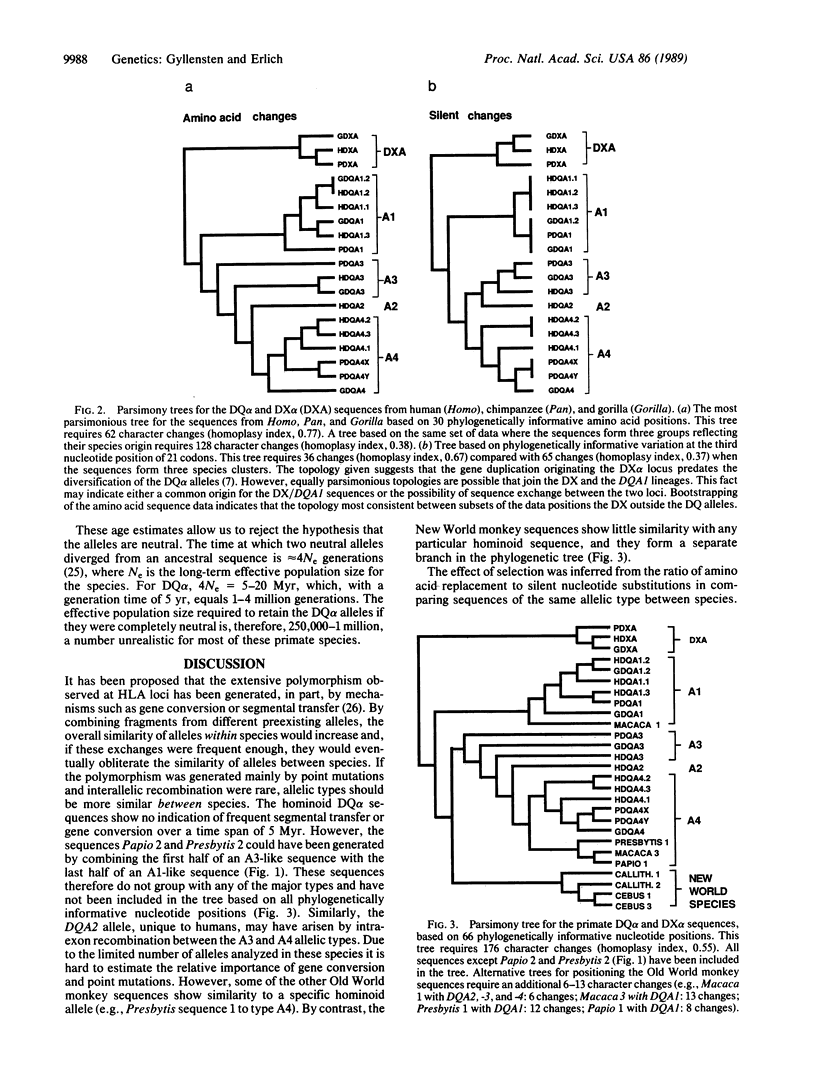
The genes encoding the human histocompatibility antigens (HLA) exhibit a remarkable degree of polymorphism as revealed by immunologic and molecular analyses. This extensive sequence polymorphism either may have been generated during the lifetime of the human species or could have arisen before speciation and been maintained in the contemporary human population by selection or, possibly, by genetic drift. These two hypotheses were examined using the polymerase chain reaction method to amplify polymorphic sequences from the DQ alpha locus, as well as the DX alpha locus, an homologous but nonexpressed locus, in a series of primates that diverged at known times. In general, the amino acid sequence of a specific human DQ alpha allelic type is more closely related to its chimpanzee or gorilla counterpart than to other human DQ alpha alleles. Phylogenetic analysis of the silent nucleotide position changes shows that the similarity of allelic types between species is due to common ancestry rather than convergent evolution. Thus, most of the polymorphism at the DQ alpha locus in the human species was already present at least 5 million years ago in the ancestral species that gave rise to the chimpanzee, gorilla, and human lineages. However, one of the DQ alpha alleles may have arisen after speciation by recombination between two ancestral alleles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden B., Klein J. Biochemical comparison of major histocompatibility complex molecules from different subspecies of Mus musculus: evidence for trans-specific evolution of alleles. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2342–2346. doi: 10.1073/pnas.79.7.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Lillie J. W., Arnot D., Grossberger D., Kappes D., Strominger J. L. Isotypic and allotypic variation of human class II histocompatibility antigen alpha-chain genes. Nature. 1984 Mar 22;308(5957):327–333. doi: 10.1038/308327a0. [DOI] [PubMed] [Google Scholar]
- Braunstein N. S., Germain R. N. Allele-specific control of Ia molecule surface expression and conformation: implications for a general model of Ia structure-function relationships. Proc Natl Acad Sci U S A. 1987 May;84(9):2921–2925. doi: 10.1073/pnas.84.9.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Bugawan T. L., Horn G. T., Long C. M., Mickelson E., Hansen J. A., Ferrara G. B., Angelini G., Erlich H. A. Analysis of HLA-DP allelic sequence polymorphism using the in vitro enzymatic DNA amplification of DP-alpha and DP-beta loci. J Immunol. 1988 Dec 1;141(11):4024–4030. [PubMed] [Google Scholar]
- Fan W. M., Kasahara M., Gutknecht J., Klein D., Mayer W. E., Jonker M., Klein J. Shared class II MHC polymorphisms between humans and chimpanzees. Hum Immunol. 1989 Oct;26(2):107–121. doi: 10.1016/0198-8859(89)90096-7. [DOI] [PubMed] [Google Scholar]
- Figueroa F., Günther E., Klein J. MHC polymorphism pre-dating speciation. Nature. 1988 Sep 15;335(6187):265–267. doi: 10.1038/335265a0. [DOI] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1579–1583. doi: 10.1073/pnas.80.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Bentley D. M., Quill H. Influence of allelic polymorphism on the assembly and surface expression of class II MHC (Ia) molecules. Cell. 1985 Nov;43(1):233–242. doi: 10.1016/0092-8674(85)90028-5. [DOI] [PubMed] [Google Scholar]
- Giles R. C., Capra J. D. Structure, function, and genetics of human class II molecules. Adv Immunol. 1985;37:1–71. doi: 10.1016/s0065-2776(08)60337-5. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Kishino H., Yano T. Man's place in Hominoidea as inferred from molecular clocks of DNA. J Mol Evol. 1987;26(1-2):132–147. doi: 10.1007/BF02111287. [DOI] [PubMed] [Google Scholar]
- Higuchi R., von Beroldingen C. H., Sensabaugh G. F., Erlich H. A. DNA typing from single hairs. Nature. 1988 Apr 7;332(6164):543–546. doi: 10.1038/332543a0. [DOI] [PubMed] [Google Scholar]
- Horn G. T., Bugawan T. L., Long C. M., Erlich H. A. Allelic sequence variation of the HLA-DQ loci: relationship to serology and to insulin-dependent diabetes susceptibility. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6012–6016. doi: 10.1073/pnas.85.16.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. C. Molecular evolution. How old is a polymorphism? Nature. 1988 Apr 14;332(6165):588–590. doi: 10.1038/332588b0. [DOI] [PubMed] [Google Scholar]
- Hughes A. L., Nei M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature. 1988 Sep 8;335(6186):167–170. doi: 10.1038/335167a0. [DOI] [PubMed] [Google Scholar]
- Klein J. Origin of major histocompatibility complex polymorphism: the trans-species hypothesis. Hum Immunol. 1987 Jul;19(3):155–162. doi: 10.1016/0198-8859(87)90066-8. [DOI] [PubMed] [Google Scholar]
- Lawlor D. A., Ward F. E., Ennis P. D., Jackson A. P., Parham P. HLA-A and B polymorphisms predate the divergence of humans and chimpanzees. Nature. 1988 Sep 15;335(6187):268–271. doi: 10.1038/335268a0. [DOI] [PubMed] [Google Scholar]
- Li W. H., Tanimura M., Sharp P. M. An evaluation of the molecular clock hypothesis using mammalian DNA sequences. J Mol Evol. 1987;25(4):330–342. doi: 10.1007/BF02603118. [DOI] [PubMed] [Google Scholar]
- Li W. H., Tanimura M. The molecular clock runs more slowly in man than in apes and monkeys. Nature. 1987 Mar 5;326(6108):93–96. doi: 10.1038/326093a0. [DOI] [PubMed] [Google Scholar]
- McConnell T. J., Talbot W. S., McIndoe R. A., Wakeland E. K. The origin of MHC class II gene polymorphism within the genus Mus. Nature. 1988 Apr 14;332(6165):651–654. doi: 10.1038/332651a0. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Geliebter J., Pfaffenbach G. M., Zeff R. A. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Pilbeam D. The descent of hominoids and hominids. Sci Am. 1984 Mar;250(3):84–96. doi: 10.1038/scientificamerican0384-84. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sakoyama Y., Hong K. J., Byun S. M., Hisajima H., Ueda S., Yaoita Y., Hayashida H., Miyata T., Honjo T. Nucleotide sequences of immunoglobulin epsilon genes of chimpanzee and orangutan: DNA molecular clock and hominoid evolution. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1080–1084. doi: 10.1073/pnas.84.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Immunological time scale for hominid evolution. Science. 1967 Dec 1;158(3805):1200–1203. doi: 10.1126/science.158.3805.1200. [DOI] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Sibley C. G., Ahlquist J. E. DNA hybridization evidence of hominoid phylogeny: results from an expanded data set. J Mol Evol. 1987;26(1-2):99–121. doi: 10.1007/BF02111285. [DOI] [PubMed] [Google Scholar]
- Stewart C. B., Schilling J. W., Wilson A. C. Adaptive evolution in the stomach lysozymes of foregut fermenters. 1987 Nov 26-Dec 2Nature. 330(6146):401–404. doi: 10.1038/330401a0. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Young J. A., Kelly A. P., Austin P. J., Carson S., Meunier H., So A., Erlich H. A., Spielman R. S., Bodmer J. Structure, sequence and polymorphism in the HLA-D region. Immunol Rev. 1985 Jul;85:5–43. doi: 10.1111/j.1600-065x.1985.tb01129.x. [DOI] [PubMed] [Google Scholar]



