Abstract
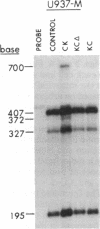
Antisense RNA is a potentially powerful tool for creating dominant negative mutations, but one of the limitations of this strategy has been the relative inefficiency of antisense transcripts in blocking target gene expression. To identify more effective target sequences, helper-free retrovirus-mediated gene transfer was used to introduce antisense RNAs complementary to multiple functional regions of the human creatine kinase B (CK-B) mRNA into U937 cells. Antisense RNA complementary to the last third of the coding and all of the noncoding regio of this mRNA is highly effective; one or two antisense transcripts is sufficient to block the expression of one CK-B mRNA. In contrast, antisense RNA from which sequences complementary to the last 17 codons and all the 3' noncoding region have been deleted has no effect on CK-B expression. Neither antisense RNA alters the abundance of the target message, processing of the primary transcript, egress of the CK-B message from the nucleus, or the polysome profile of CK-B mRNA in sucrose gradients. These results point to a direct effect of the antisense transcript on translation and suggest that this effect may be explained at least in part by an inhibition of elongation or termination as a consequence of the duplex formed in the distal coding and/or 3' noncoding region.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Both subunits of rat liver ferritin are regulated at a translational level by iron induction. Nucleic Acids Res. 1986 Jan 24;14(2):915–927. doi: 10.1093/nar/14.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. D., Adams S. L. Characterization of the translational control mechanism preventing synthesis of alpha 2(I) collagen in chicken vertebral chondroblasts. J Biol Chem. 1987 Oct 25;262(30):14806–14814. [PubMed] [Google Scholar]
- Berry J. O., Carr J. P., Klessig D. F. mRNAs encoding ribulose-1,5-bisphosphate carboxylase remain bound to polysomes but are not translated in amaranth seedlings transferred to darkness. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4190–4194. doi: 10.1073/pnas.85.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choudary P. V., Tsuji S., Martin B. M., Guild B. C., Mulligan R. C., Murray G. J., Barranger J. A., Ginns E. I. The molecular biology of Gaucher disease and the potential for gene therapy. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):1047–1052. doi: 10.1101/sqb.1986.051.01.121. [DOI] [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T. E., Nellen W., Gomer R. H., Firtel R. A. Phenocopy of discoidin I-minus mutants by antisense transformation in Dictyostelium. Cell. 1985 Dec;43(3 Pt 2):633–641. doi: 10.1016/0092-8674(85)90235-1. [DOI] [PubMed] [Google Scholar]
- Daouk G. H., Kaddurah-Daouk R., Putney S., Kingston R., Schimmel P. Isolation of a functional human gene for brain creatine kinase. J Biol Chem. 1988 Feb 15;263(5):2442–2446. [PubMed] [Google Scholar]
- Daugherty B. L., Hotta K., Kumar C., Ahn Y. H., Zhu J. D., Pestka S. Antisense RNA: effect of ribosome binding sites, target location, size, and concentration on the translation of specific mRNA molecules. Gene Anal Tech. 1989 Jan-Feb;6(1):1–16. doi: 10.1016/0735-0651(89)90007-1. [DOI] [PubMed] [Google Scholar]
- Ellison M. J., Kelleher R. J., 3rd, Rich A. Thermal regulation of beta-galactosidase synthesis using anti-sense RNA directed against the coding portion of the mRNA. J Biol Chem. 1985 Aug 5;260(16):9085–9087. [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Anderson S. J., Larrick J. W. In vitro activation of a human macrophage-like cell line. Nature. 1979 May 24;279(5711):328–331. doi: 10.1038/279328a0. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Leech A. R., Beis I., Newsholme E. A. Radiochemical assays for creatine kinase and arginine kinase using rapid ion exchange separations. Anal Biochem. 1978 Oct 15;90(2):561–575. doi: 10.1016/0003-2697(78)90150-1. [DOI] [PubMed] [Google Scholar]
- Loike J. D., Kozler V. F., Silverstein S. C. Creatine kinase expression and creatine phosphate accumulation are developmentally regulated during differentiation of mouse and human monocytes. J Exp Med. 1984 Mar 1;159(3):746–757. doi: 10.1084/jem.159.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. Inhibition of heat shock protein synthesis by heat-inducible antisense RNA. Proc Natl Acad Sci U S A. 1986 Jan;83(2):399–403. doi: 10.1073/pnas.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe S. H. Antisense RNA inhibits splicing of pre-mRNA in vitro. EMBO J. 1988 Aug;7(8):2523–2532. doi: 10.1002/j.1460-2075.1988.tb03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenbrock T., Wille W. The 3' non-coding region of the mouse brain B creatine kinase mRNA: a sequence with exceptional homology among species. Nucleic Acids Res. 1986 Nov 11;14(21):8690–8690. doi: 10.1093/nar/14.21.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakin S. H., Liebhaber S. A. Destabilization of messenger RNA/complementary DNA duplexes by the elongating 80 S ribosome. J Biol Chem. 1986 Dec 5;261(34):16018–16025. [PubMed] [Google Scholar]
- Skadsen R. W., Scandalios J. G. Translational control of photo-induced expression of the Cat2 catalase gene during leaf development in maize. Proc Natl Acad Sci U S A. 1987 May;84(9):2785–2789. doi: 10.1073/pnas.84.9.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. C., Aurelian L., Reddy M. P., Miller P. S., Ts'o P. O. Antiviral effect of an oligo(nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc Natl Acad Sci U S A. 1986 May;83(9):2787–2791. doi: 10.1073/pnas.83.9.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Huarte J., Belin D., Vassalli A., Rickles R. J., Vassalli J. D. Antisense RNA directed against the 3' noncoding region prevents dormant mRNA activation in mouse oocytes. Science. 1988 Aug 5;241(4866):680–684. doi: 10.1126/science.2456615. [DOI] [PubMed] [Google Scholar]
- Theodorakis N. G., Banerji S. S., Morimoto R. I. HSP70 mRNA translation in chicken reticulocytes is regulated at the level of elongation. J Biol Chem. 1988 Oct 5;263(28):14579–14585. [PubMed] [Google Scholar]
- Tscherne J. S., Pestka S. Inhibition of protein synthesis in intact HeLa cells. Antimicrob Agents Chemother. 1975 Oct;8(4):479–487. doi: 10.1128/aac.8.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin S. L., Walter P. Ribosome pausing and stacking during translation of a eukaryotic mRNA. EMBO J. 1988 Nov;7(11):3559–3569. doi: 10.1002/j.1460-2075.1988.tb03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormington W. M. Stable repression of ribosomal protein L1 synthesis in Xenopus oocytes by microinjection of antisense RNA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8639–8643. doi: 10.1073/pnas.83.22.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]