Abstract
Human erythrocyte glucose-6-phosphate dehydrogenase is normally quite stable in the presence of 10 microM NADP+. Certain glucose-6-phosphate dehydrogenase variants lose virtually all their activity at this concentration of NADP+ but are reactivated by 200 microM NADP+. Such variants presumably have a defect in their NADP+-binding site. We analyzed the sequence of cDNA or genomic DNA from seven unrelated patients with hemolytic anemia due to the inheritance of variants that are reactivated by NADP+. Six patients had substitutions of one of three adjacent amino acids, and the seventh patient had another amino acid substitution 23 residues downstream. These amino acids are highly conserved, all being present in rat and all but one being found also in Drosophila. The anomalous electrophoretic behavior of some of the variants can be explained by their loss of ability to bind NADP+. We conclude that the region in which these mutations occur defines the binding domain for NADP+ and that binding NADP+ that has been designated as "structural" and as "catalytic" probably occurs at the same site.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., Collins Z. Hybridization of Glucose-6-Phosphate Dehydrogenase from Rat and Human Erythrocytes. Science. 1965 Dec 3;150(3701):1306–1307. doi: 10.1126/science.150.3701.1306. [DOI] [PubMed] [Google Scholar]
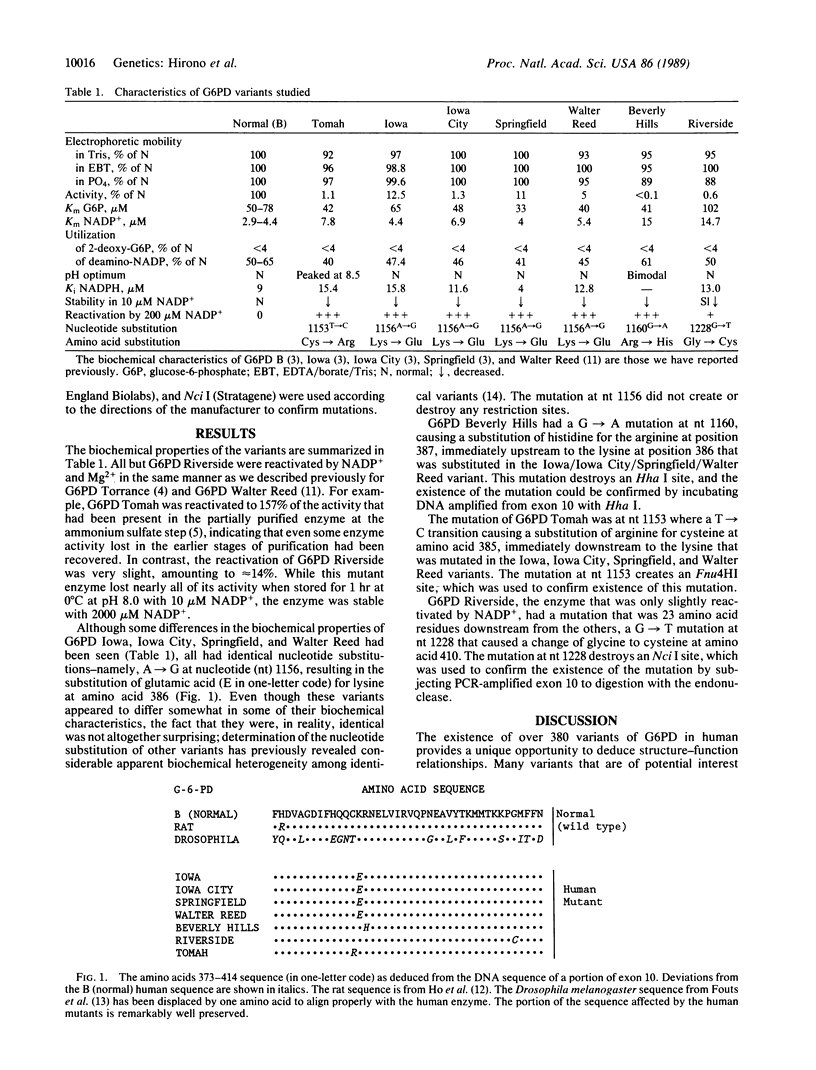
- Beutler E. Glucose-6-phosphate dehydrogenase: new perspectives. Blood. 1989 May 1;73(6):1397–1401. [PubMed] [Google Scholar]
- Beutler E., Hartman K., Gelbart T., Forman L. G-6-PD Walter Reed: possible insight into "structural" NADP in G-6-PD. Am J Hematol. 1986 Sep;23(1):25–30. doi: 10.1002/ajh.2830230105. [DOI] [PubMed] [Google Scholar]
- Beutler E., Kuhl W., Vives-Corrons J. L., Prchal J. T. Molecular heterogeneity of glucose-6-phosphate dehydrogenase A-. Blood. 1989 Nov 15;74(7):2550–2555. [PubMed] [Google Scholar]
- Beutler E., Yoshida A. Genetic variation of glucose-6-phosphate dehydrogenase: a catalog and future prospects. Medicine (Baltimore) 1988 Sep;67(5):311–334. doi: 10.1097/00005792-198809000-00003. [DOI] [PubMed] [Google Scholar]
- Chehab F. F., Doherty M., Cai S. P., Kan Y. W., Cooper S., Rubin E. M. Detection of sickle cell anaemia and thalassaemias. Nature. 1987 Sep 24;329(6137):293–294. doi: 10.1038/329293b0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- De Flora A., Morelli A., Giuliano F. Human erythrocyte glucose 6-phosphate dehydrogenase. Content of bound coenzyme. Biochem Biophys Res Commun. 1974 Jul 10;59(1):406–413. doi: 10.1016/s0006-291x(74)80221-4. [DOI] [PubMed] [Google Scholar]
- De Vita G., Alcalay M., Sampietro M., Cappelini M. D., Fiorelli G., Toniolo D. Two point mutations are responsible for G6PD polymorphism in Sardinia. Am J Hum Genet. 1989 Feb;44(2):233–240. [PMC free article] [PubMed] [Google Scholar]
- Fouts D., Ganguly R., Gutierrez A. G., Lucchesi J. C., Manning J. E. Nucleotide sequence of the Drosophila glucose-6-phosphate dehydrogenase gene and comparison with the homologous human gene. Gene. 1988 Mar 31;63(2):261–275. doi: 10.1016/0378-1119(88)90530-6. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Howard A. J., Crapo J. D. Cloning and sequence of a cDNA encoding rat glucose-6-phosphate dehydrogenase. Nucleic Acids Res. 1988 Aug 11;16(15):7746–7746. doi: 10.1093/nar/16.15.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRKMAN H. N., HENDRICKSON E. M. Glucose 6-phosphate dehydrogenase from human erythrocytes. II. Subactive states of the enzyme from normal persons. J Biol Chem. 1962 Jul;237:2371–2376. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. R., Beutler E. Hereditary hemolytic anemia due to glucose-6-phosphate dehydrogenase Torrance: a new variant. J Lab Clin Med. 1969 Apr;73(4):657–667. [PubMed] [Google Scholar]
- Vulliamy T. J., D'Urso M., Battistuzzi G., Estrada M., Foulkes N. S., Martini G., Calabro V., Poggi V., Giordano R., Town M. Diverse point mutations in the human glucose-6-phosphate dehydrogenase gene cause enzyme deficiency and mild or severe hemolytic anemia. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5171–5175. doi: 10.1073/pnas.85.14.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Hoagland V. D., Jr Active molecular unit and NADP content of human glucose 6-phosphate dehydrogenase. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1167–1172. doi: 10.1016/0006-291x(70)90917-4. [DOI] [PubMed] [Google Scholar]
- Yoshida A. Subunit structure of human glucose 6-phosphate dehydrogenase and its genetic implication. Biochem Genet. 1968 Nov;2(3):237–243. doi: 10.1007/BF01474763. [DOI] [PubMed] [Google Scholar]



