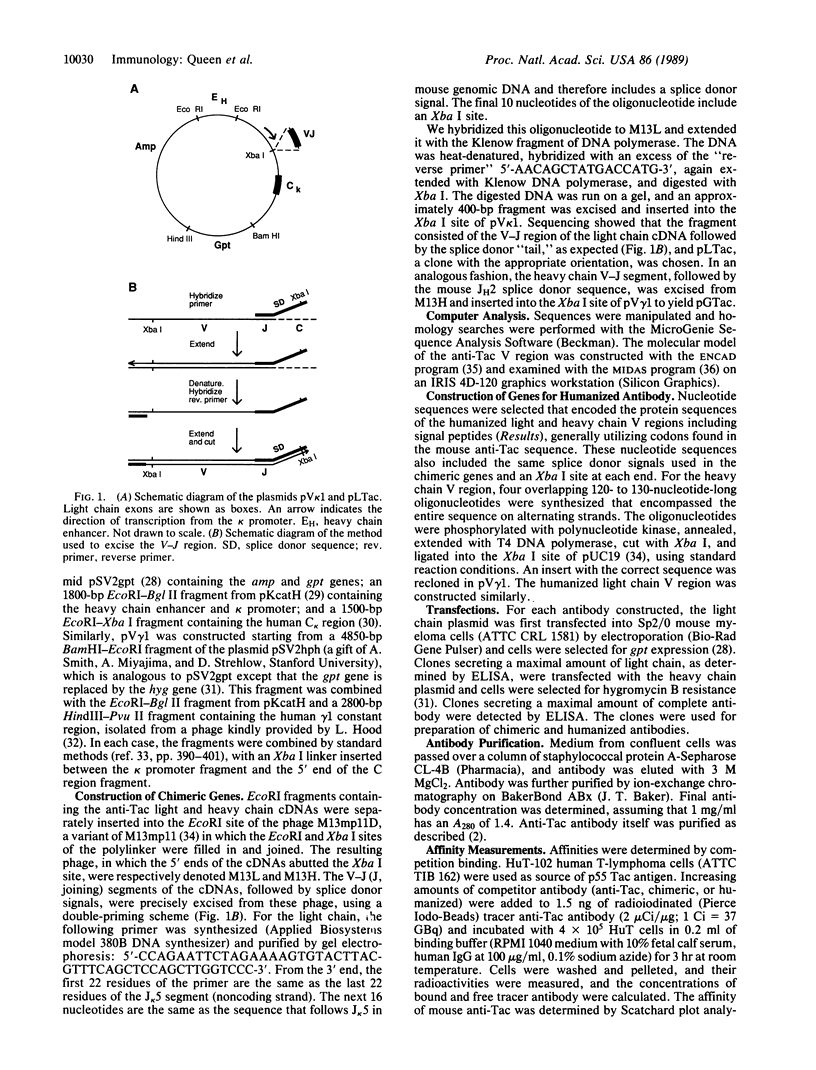
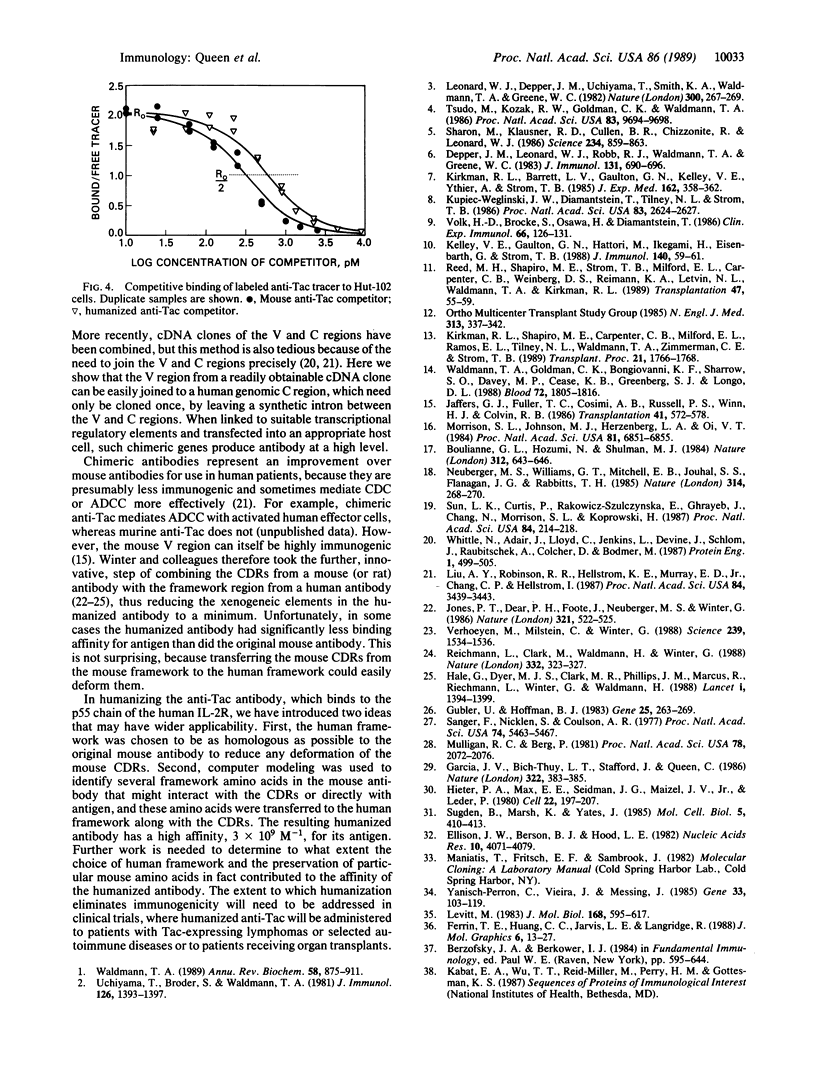
Abstract
The anti-Tac monoclonal antibody is known to bind to the p55 chain of the human interleukin 2 receptor and to inhibit proliferation of T cells by blocking interleukin 2 binding. However, use of anti-Tac as an immunosuppressant drug would be impaired by the human immune response against this murine antibody. We have therefore constructed a "humanized" antibody by combining the complementarity-determining regions (CDRs) of the anti-Tac antibody with human framework and constant regions. The human framework regions were chosen to maximize homology with the anti-Tac antibody sequence. In addition, a computer model of murine anti-Tac was used to identify several amino acids which, while outside the CDRs, are likely to interact with the CDRs or antigen. These mouse amino acids were also retained in the humanized antibody. The humanized anti-Tac antibody has an affinity for p55 of 3 x 10(9) M-1, about 1/3 that of murine anti-Tac.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulianne G. L., Hozumi N., Shulman M. J. Production of functional chimaeric mouse/human antibody. Nature. 1984 Dec 13;312(5995):643–646. doi: 10.1038/312643a0. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Robb R. J., Waldmann T. A., Greene W. C. Blockade of the interleukin-2 receptor by anti-Tac antibody: inhibition of human lymphocyte activation. J Immunol. 1983 Aug;131(2):690–696. [PubMed] [Google Scholar]
- Ellison J. W., Berson B. J., Hood L. E. The nucleotide sequence of a human immunoglobulin C gamma1 gene. Nucleic Acids Res. 1982 Jul 10;10(13):4071–4079. doi: 10.1093/nar/10.13.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. V., Bich-Thuy L. T., Stafford J., Queen C. Synergism between immunoglobulin enhancers and promoters. Nature. 1986 Jul 24;322(6077):383–385. doi: 10.1038/322383a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hale G., Dyer M. J., Clark M. R., Phillips J. M., Marcus R., Riechmann L., Winter G., Waldmann H. Remission induction in non-Hodgkin lymphoma with reshaped human monoclonal antibody CAMPATH-1H. Lancet. 1988 Dec 17;2(8625):1394–1399. doi: 10.1016/s0140-6736(88)90588-0. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Max E. E., Seidman J. G., Maizel J. V., Jr, Leder P. Cloned human and mouse kappa immunoglobulin constant and J region genes conserve homology in functional segments. Cell. 1980 Nov;22(1 Pt 1):197–207. doi: 10.1016/0092-8674(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Jaffers G. J., Fuller T. C., Cosimi A. B., Russell P. S., Winn H. J., Colvin R. B. Monoclonal antibody therapy. Anti-idiotypic and non-anti-idiotypic antibodies to OKT3 arising despite intense immunosuppression. Transplantation. 1986 May;41(5):572–578. doi: 10.1097/00007890-198605000-00004. [DOI] [PubMed] [Google Scholar]
- Jones P. T., Dear P. H., Foote J., Neuberger M. S., Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. 1986 May 29-Jun 4Nature. 321(6069):522–525. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- Kelley V. E., Gaulton G. N., Hattori M., Ikegami H., Eisenbarth G., Strom T. B. Anti-interleukin 2 receptor antibody suppresses murine diabetic insulitis and lupus nephritis. J Immunol. 1988 Jan 1;140(1):59–61. [PubMed] [Google Scholar]
- Kirkman R. L., Barrett L. V., Gaulton G. N., Kelley V. E., Ythier A., Strom T. B. Administration of an anti-interleukin 2 receptor monoclonal antibody prolongs cardiac allograft survival in mice. J Exp Med. 1985 Jul 1;162(1):358–362. doi: 10.1084/jem.162.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman R. L., Shapiro M. E., Carpenter C. B., Milford E. L., Ramos E. L., Tilney N. L., Waldmann T. A., Zimmerman C. E., Strom T. B. Early experience with anti-Tac in clinical renal transplantation. Transplant Proc. 1989 Feb;21(1 Pt 2):1766–1768. [PubMed] [Google Scholar]
- Kupiec-Weglinski J. W., Diamantstein T., Tilney N. L., Strom T. B. Therapy with monoclonal antibody to interleukin 2 receptor spares suppressor T cells and prevents or reverses acute allograft rejection in rats. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2624–2627. doi: 10.1073/pnas.83.8.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Levitt M. Molecular dynamics of native protein. I. Computer simulation of trajectories. J Mol Biol. 1983 Aug 15;168(3):595–617. doi: 10.1016/s0022-2836(83)80304-0. [DOI] [PubMed] [Google Scholar]
- Liu A. Y., Robinson R. R., Hellström K. E., Murray E. D., Jr, Chang C. P., Hellström I. Chimeric mouse-human IgG1 antibody that can mediate lysis of cancer cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3439–3443. doi: 10.1073/pnas.84.10.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. L., Johnson M. J., Herzenberg L. A., Oi V. T. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6851–6855. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Mitchell E. B., Jouhal S. S., Flanagan J. G., Rabbitts T. H. A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature. 1985 Mar 21;314(6008):268–270. doi: 10.1038/314268a0. [DOI] [PubMed] [Google Scholar]
- Reed M. H., Shapiro M. E., Strom T. B., Milford E. L., Carpenter C. B., Weinberg D. S., Reimann K. A., Letvin N. L., Waldmann T. A., Kirkman R. L. Prolongation of primate renal allograft survival by anti-Tac, an anti-human IL-2 receptor monoclonal antibody. Transplantation. 1989 Jan;47(1):55–59. doi: 10.1097/00007890-198901000-00013. [DOI] [PubMed] [Google Scholar]
- Riechmann L., Clark M., Waldmann H., Winter G. Reshaping human antibodies for therapy. Nature. 1988 Mar 24;332(6162):323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. K., Curtis P., Rakowicz-Szulczynska E., Ghrayeb J., Chang N., Morrison S. L., Koprowski H. Chimeric antibody with human constant regions and mouse variable regions directed against carcinoma-associated antigen 17-1A. Proc Natl Acad Sci U S A. 1987 Jan;84(1):214–218. doi: 10.1073/pnas.84.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Verhoeyen M., Milstein C., Winter G. Reshaping human antibodies: grafting an antilysozyme activity. Science. 1988 Mar 25;239(4847):1534–1536. doi: 10.1126/science.2451287. [DOI] [PubMed] [Google Scholar]
- Volk H. D., Brocke S., Osawa H., Diamantstein T. Effects of in-vivo administration of a monoclonal antibody specific for the interleukin-2 receptor on the acute graft-versus-host reaction in mice. Clin Exp Immunol. 1986 Oct;66(1):126–131. [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Goldman C. K., Bongiovanni K. F., Sharrow S. O., Davey M. P., Cease K. B., Greenberg S. J., Longo D. L. Therapy of patients with human T-cell lymphotrophic virus I-induced adult T-cell leukemia with anti-Tac, a monoclonal antibody to the receptor for interleukin-2. Blood. 1988 Nov;72(5):1805–1816. [PubMed] [Google Scholar]
- Waldmann T. A. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875–911. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- Whittle N., Adair J., Lloyd C., Jenkins L., Devine J., Schlom J., Raubitschek A., Colcher D., Bodmer M. Expression in COS cells of a mouse-human chimaeric B72.3 antibody. Protein Eng. 1987 Dec;1(6):499–505. doi: 10.1093/protein/1.6.499. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]