Abstract
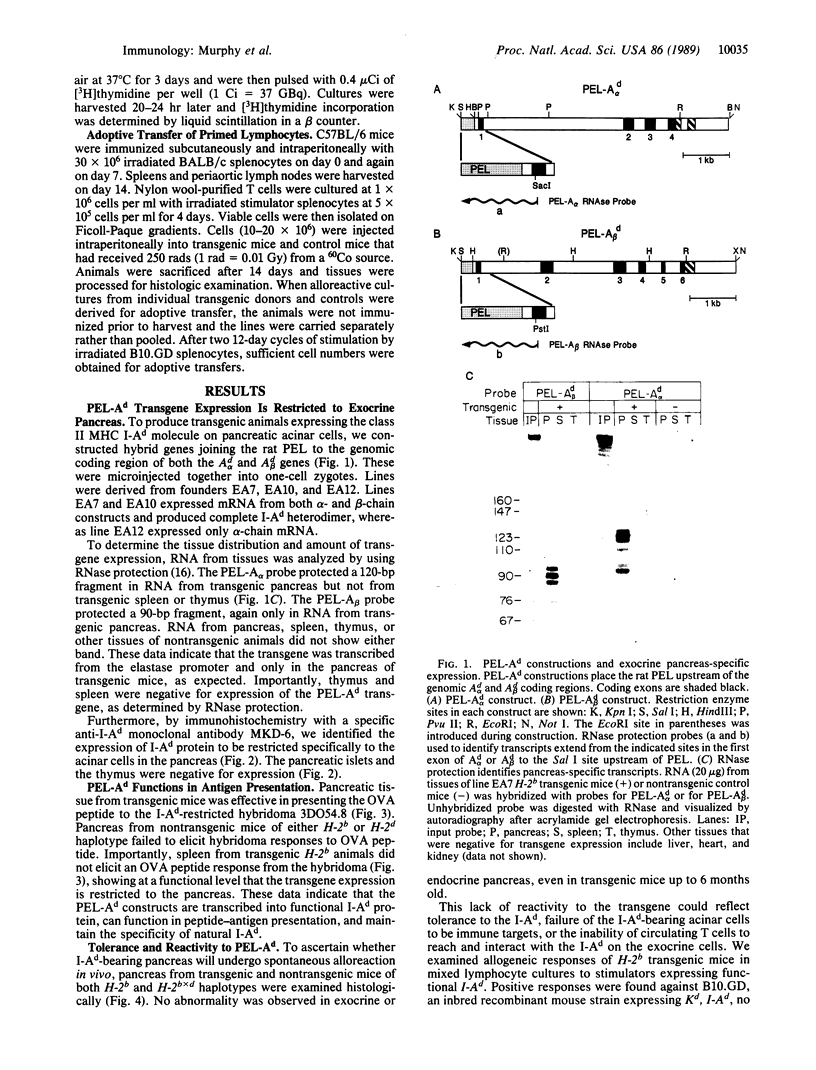
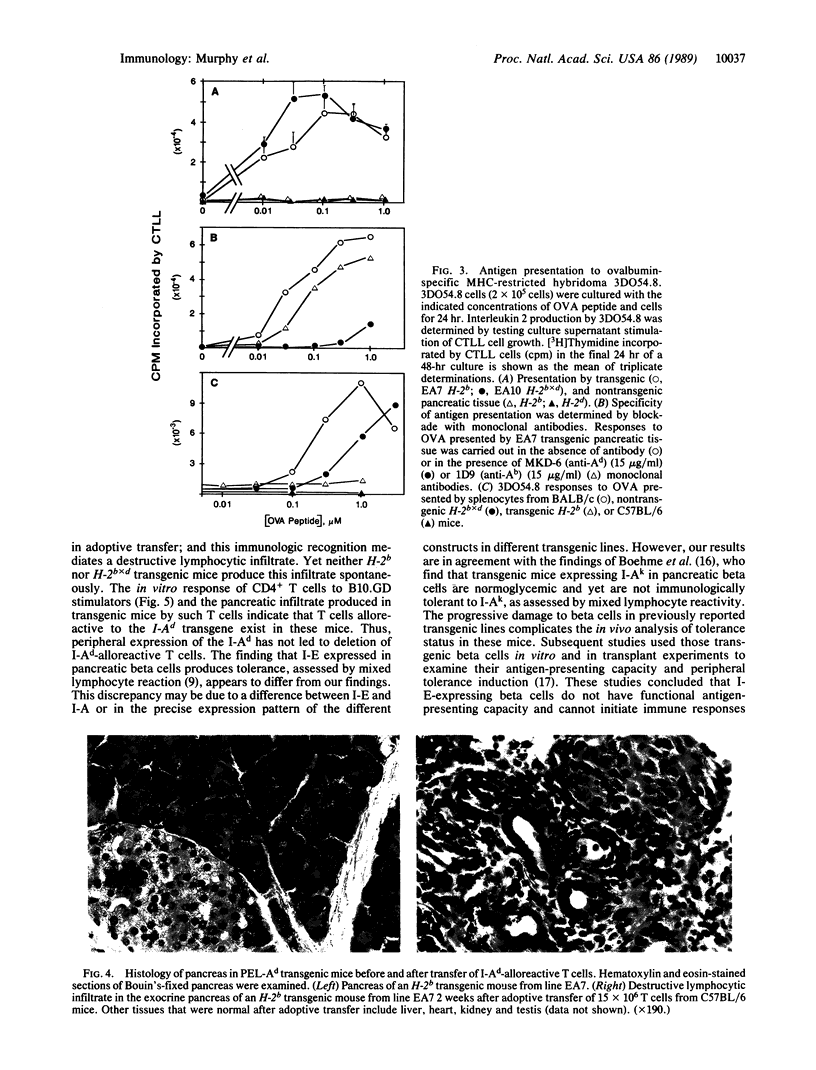
To examine the effects of aberrant expression of class II major histocompatibility complex (MHC) proteins on tolerance development, transgenic mice expressing the I-Ad genes under control of the pancreatic elastase promoter were produced. Such transgenic mice express I-Ad exclusively on exocrine pancreas, without expression in thymus or by lymphocytes. No spontaneous development of autoimmune reactivity toward exocrine pancreas was found in transgene-expressing mice of an H-2b background even though such mice could produce in vitro allogeneic responses against I-Ad. When T cells from nontransgenic H-2b mice as well as transgenic H-2b mice were activated in vitro by I-Ad allogeneic stimulator cells and transferred to transgenic mice, an intense, destructive lymphocytic infiltrate specific for exocrine pancreas developed. These findings suggest that aberrant class II MHC expression alone may not trigger autoimmune reactions. Rather, the unresponsiveness to allogenic class II MHC may result from the inability of exocrine pancreas to initiate primary responses by T cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J., Campbell I. L., Morahan G., Mandel T. E., Harrison L. C., Miller J. F. Diabetes in transgenic mice resulting from over-expression of class I histocompatibility molecules in pancreatic beta cells. Nature. 1988 Jun 9;333(6173):529–533. doi: 10.1038/333529a0. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Böhme J., Haskins K., Stecha P., van Ewijk W., LeMeur M., Gerlinger P., Benoist C., Mathis D. Transgenic mice with I-A on islet cells are normoglycemic but immunologically intolerant. Science. 1989 Jun 9;244(4909):1179–1183. doi: 10.1126/science.2499048. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crimmins D. L., Gorka J., Thoma R. S., Schwartz B. D. Peptide characterization with a sulfoethyl aspartamide column. J Chromatogr. 1988 Jun 29;443:63–71. doi: 10.1016/s0021-9673(00)94783-6. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Swift G. H., Ornitz D. M., Quaife C. J., Palmiter R. D., Brinster R. L., MacDonald R. J. The rat elastase I regulatory element is an enhancer that directs correct cell specificity and developmental onset of expression in transgenic mice. Mol Cell Biol. 1987 Aug;7(8):2956–2967. doi: 10.1128/mcb.7.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Pardoll D. M., Mizuguchi J., Quill H., Schwartz R. H. T-cell unresponsiveness in vivo and in vitro: fine specificity of induction and molecular characterization of the unresponsive state. Immunol Rev. 1987 Feb;95:113–135. doi: 10.1111/j.1600-065x.1987.tb00502.x. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Lo D., Burkly L. C., Flavell R. A., Palmiter R. D., Brinster R. L. Tolerance in transgenic mice expressing class II major histocompatibility complex on pancreatic acinar cells. J Exp Med. 1989 Jul 1;170(1):87–104. doi: 10.1084/jem.170.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D., Burkly L. C., Widera G., Cowing C., Flavell R. A., Palmiter R. D., Brinster R. L. Diabetes and tolerance in transgenic mice expressing class II MHC molecules in pancreatic beta cells. Cell. 1988 Apr 8;53(1):159–168. doi: 10.1016/0092-8674(88)90497-7. [DOI] [PubMed] [Google Scholar]
- Markmann J., Lo D., Naji A., Palmiter R. D., Brinster R. L., Heber-Katz E. Antigen presenting function of class II MHC expressing pancreatic beta cells. Nature. 1988 Dec 1;336(6198):476–479. doi: 10.1038/336476a0. [DOI] [PubMed] [Google Scholar]
- Morahan G., Brennan F. E., Bhathal P. S., Allison J., Cox K. O., Miller J. F. Expression in transgenic mice of class I histocompatibility antigens controlled by the metallothionein promoter. Proc Natl Acad Sci U S A. 1989 May;86(10):3782–3786. doi: 10.1073/pnas.86.10.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol-Borrell R., Bottazzo G. F. Puzzling diabetic transgenic mice: a lesson for human type 1 diabetes? Immunol Today. 1988 Oct;9(10):303–306. doi: 10.1016/0167-5699(88)91322-9. [DOI] [PubMed] [Google Scholar]
- Sarvetnick N., Liggitt D., Pitts S. L., Hansen S. E., Stewart T. A. Insulin-dependent diabetes mellitus induced in transgenic mice by ectopic expression of class II MHC and interferon-gamma. Cell. 1988 Mar 11;52(5):773–782. doi: 10.1016/0092-8674(88)90414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- Zamoyska R., Waldmann H., Matzinger P. Peripheral tolerance mechanisms prevent the development of autoreactive T cells in chimeras grafted with two minor incompatible thymuses. Eur J Immunol. 1989 Jan;19(1):111–117. doi: 10.1002/eji.1830190118. [DOI] [PubMed] [Google Scholar]