Abstract
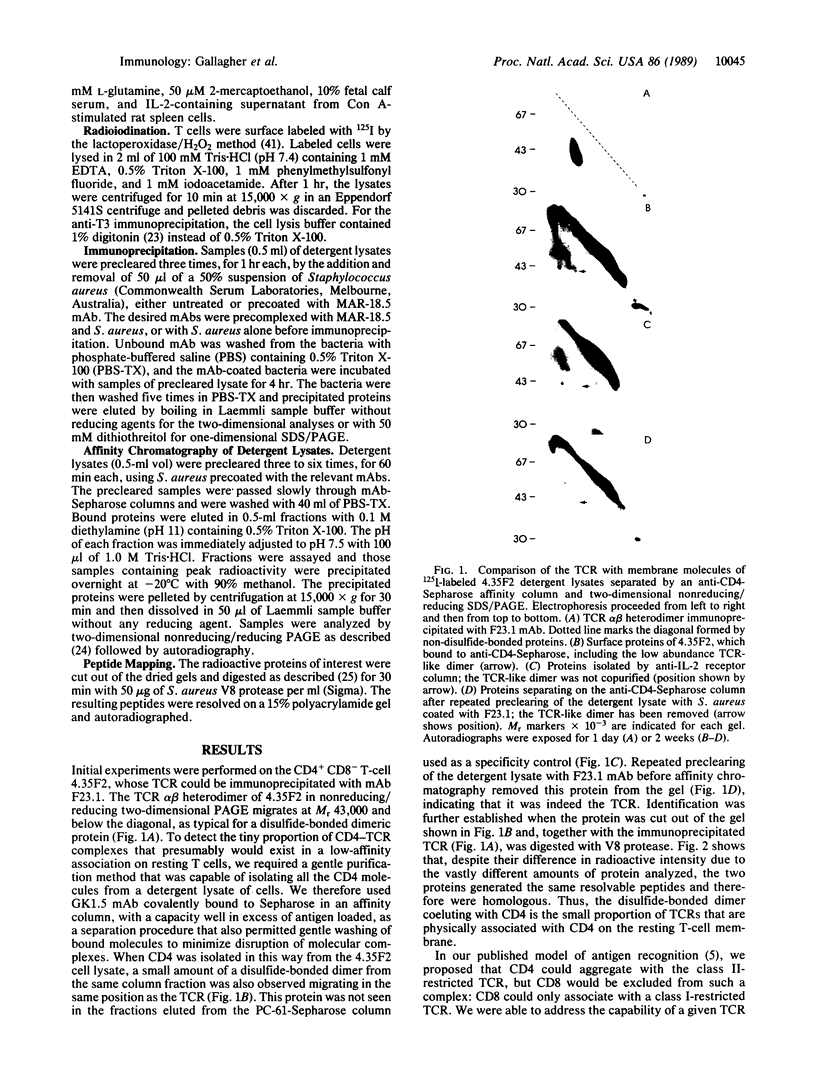
The expression of the cell-surface glycoproteins CD4 and CD8 on functionally mature T cells is usually mutually exclusive and correlates with class II and class I major histocompatibility complex (MHC) restriction, respectively. CD4 and CD8 function by binding to class II and class I MHC molecules on the antigen-presenting cell (APC), thereby increasing the adhesion between the T cell and the APC. From antibody-blocking studies and from cocapping and comodulation experiments, CD4 and CD8 come into close physical contact with their appropriately restricted T-cell receptor (TCR) at the time of antigen recognition. By the use of affinity chromatography followed by two-dimensional diagonal gel electrophoresis, we have identified a Mr 43,000 disulfide-bonded heterodimer copurifying with CD4. This protein was identified as the TCR by its removal after preclearing with the anti-TCR antibody F23.1 and by its generation after protease digestion of the same peptides as the TCR from this clone. When CD4 and CD8 were similarly isolated from an unusual CD4+ CD8+ class II-restricted T-cell clone, the TCR was identified as associating with either accessory molecule in the absence of activation. Therefore, CD4 and CD8 do not distinguish between class I- and class II-restricted TCRs in their ability to form membrane complexes, indicating a need for both the TCR and its associated accessory molecule to recognize the same individual MHC molecule on the APC to optimize TCR triggering.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Blue M. L., Schlossman S. F. Comodulation of CD3 and CD4. Evidence for a specific association between CD4 and approximately 5% of the CD3:T cell receptor complexes on helper T lymphocytes. J Immunol. 1988 Mar 15;140(6):1732–1737. [PubMed] [Google Scholar]
- Ballhausen W. G., Reske-Kunz A. B., Tourvieille B., Ohashi P. S., Parnes J. R., Mak T. W. Acquisition of an additional antigen specificity after mouse CD4 gene transfer into a T helper hybridoma. J Exp Med. 1988 Apr 1;167(4):1493–1498. doi: 10.1084/jem.167.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber E. K., Dasgupta J. D., Schlossman S. F., Trevillyan J. M., Rudd C. E. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci U S A. 1989 May;86(9):3277–3281. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. A., Kappler J. W., Marrack P. T-cell specificity and repertoire. Immunol Rev. 1988 Jan;101:5–19. doi: 10.1111/j.1600-065x.1988.tb00730.x. [DOI] [PubMed] [Google Scholar]
- Blue M. L., Craig K. A., Anderson P., Branton K. R., Jr, Schlossman S. F. Evidence for specific association between class I major histocompatibility antigens and the CD8 molecules of human suppressor/cytotoxic cells. Cell. 1988 Jul 29;54(3):413–421. doi: 10.1016/0092-8674(88)90204-8. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Hyman R., Trowbridge I., Singer S. J. Participation of histocompatibility antigens in capping of molecularly independent cell surface components by their specific antibodies. Proc Natl Acad Sci U S A. 1978 May;75(5):2406–2410. doi: 10.1073/pnas.75.5.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce N. W., Jönsson J. I., Emmrich F., Eichmann K. Heterologous cross-linking of Lyt-2 (CD8) to the alpha beta-T cell receptor is more effective in T cell activation than homologous alpha beta-T cell receptor cross-linking. J Immunol. 1988 Nov 1;141(9):2882–2888. [PubMed] [Google Scholar]
- Bushkin Y., Demaria S., Le J. M., Schwab R. Physical association between the CD8 and HLA class I molecules on the surface of activated human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3985–3989. doi: 10.1073/pnas.85.11.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Haas W., Zamoyska R., Parnes J., Steinmetz M., von Boehmer H. Transfection of the CD8 gene enhances T-cell recognition. Nature. 1987 Apr 2;326(6112):510–511. doi: 10.1038/326510a0. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Kanz L., Eichmann K. Cross-linking of the T cell receptor complex with the subset-specific differentiation antigen stimulates interleukin 2 receptor expression in human CD4 and CD8 T cells. Eur J Immunol. 1987 Apr;17(4):529–534. doi: 10.1002/eji.1830170415. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Strittmatter U., Eichmann K. Synergism in the activation of human CD8 T cells by cross-linking the T-cell receptor complex with the CD8 differentiation antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8298–8302. doi: 10.1073/pnas.83.21.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas de St Groth B., Gallagher P. F., Miller J. F. Involvement of Lyt-2 and L3T4 in activation of hapten-specific Lyt-2+ L3T4+ T-cell clones. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2594–2598. doi: 10.1073/pnas.83.8.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabert J., Langlet C., Zamoyska R., Parnes J. R., Schmitt-Verhulst A. M., Malissen B. Reconstitution of MHC class I specificity by transfer of the T cell receptor and Lyt-2 genes. Cell. 1987 Aug 14;50(4):545–554. doi: 10.1016/0092-8674(87)90027-4. [DOI] [PubMed] [Google Scholar]
- Gallagher P. F., Fazekas de St Groth B., Miller J. F. Stable expression of Lyt-2 homodimers on L3T4+ T cell clones. Eur J Immunol. 1986 Nov;16(11):1413–1417. doi: 10.1002/eji.1830161116. [DOI] [PubMed] [Google Scholar]
- Goding J. W., Harris A. W. Subunit structure of cell surface proteins: disulfide bonding in antigen receptors, Ly-2/3 antigens, and transferrin receptors of murine T and B lymphocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4530–4534. doi: 10.1073/pnas.78.7.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Structural studies of murine lymphocyte surface IgD. J Immunol. 1980 May;124(5):2082–2088. [PubMed] [Google Scholar]
- Greenstein J. L., Kappler J., Marrack P., Burakoff S. J. The role of L3T4 in recognition of Ia by a cytotoxic, H-2Dd-specific T cell hybridoma. J Exp Med. 1984 Apr 1;159(4):1213–1224. doi: 10.1084/jem.159.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Haque S., Smith L. A., Saizawa K. The role of the murine L3T4 molecule in T cell activation: differential effects of anti-L3T4 on activation by monoclonal anti-receptor antibodies. J Mol Cell Immunol. 1987;3(2):121–131. [PubMed] [Google Scholar]
- Jones B., Khavari P. A., Conrad P. J., Janeway C. A., Jr Differential effects of antibodies to Lyt-2 and L3T4 on cytolysis by cloned, Ia-restricted T cells expressing both proteins. J Immunol. 1987 Jul 15;139(2):380–384. [PubMed] [Google Scholar]
- Kupfer A., Singer S. J., Janeway C. A., Jr, Swain S. L. Coclustering of CD4 (L3T4) molecule with the T-cell receptor is induced by specific direct interaction of helper T cells and antigen-presenting cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5888–5892. doi: 10.1073/pnas.84.16.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. Molecular dynamics in the membranes of helper T cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8216–8220. doi: 10.1073/pnas.85.21.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Gutman G. A., Lewis D. E., Griswold S. T., Warner N. L. Monoclonal antibodies against rat immunoglobulin kappa chains. Hybridoma. 1982;1(2):125–131. doi: 10.1089/hyb.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Tonks N. K., Fischer E. H., Clark E. A. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8628–8632. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. Adv Immunol. 1986;38:1–30. doi: 10.1016/s0065-2776(08)60005-x. [DOI] [PubMed] [Google Scholar]
- Norment A. M., Salter R. D., Parham P., Engelhard V. H., Littman D. R. Cell-cell adhesion mediated by CD8 and MHC class I molecules. Nature. 1988 Nov 3;336(6194):79–81. doi: 10.1038/336079a0. [DOI] [PubMed] [Google Scholar]
- O'Neill H. C., McGrath M. S., Allison J. P., Weissman I. L. A subset of T cell receptors associated with L3T4 molecules mediates C6VL leukemia cell binding of its cognate retrovirus. Cell. 1987 Apr 10;49(1):143–151. doi: 10.1016/0092-8674(87)90764-1. [DOI] [PubMed] [Google Scholar]
- Oettgen H. C., Pettey C. L., Maloy W. L., Terhorst C. A T3-like protein complex associated with the antigen receptor on murine T cells. Nature. 1986 Mar 20;320(6059):272–275. doi: 10.1038/320272a0. [DOI] [PubMed] [Google Scholar]
- Owens T., Fazekas de St Groth B., Miller J. F. Coaggregation of the T-cell receptor with CD4 and other T-cell surface molecules enhances T-cell activation. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9209–9213. doi: 10.1073/pnas.84.24.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T., Fazekas de St Groth B. Participation of L3T4 in T cell activation in the absence of class II major histocompatibility complex antigens. Inhibition by anti-L3T4 antibodies is a function both of epitope density and mode of presentation of anti-receptor antibody. J Immunol. 1987 Apr 15;138(8):2402–2409. [PubMed] [Google Scholar]
- Rivas A., Takada S., Koide J., Sonderstrup-McDevitt G., Engleman E. G. CD4 molecules are associated with the antigen receptor complex on activated but not resting T cells. J Immunol. 1988 May 1;140(9):2912–2918. [PubMed] [Google Scholar]
- Rojo J. M., Saizawa K., Janeway C. A., Jr Physical association of CD4 and the T-cell receptor can be induced by anti-T-cell receptor antibodies. Proc Natl Acad Sci U S A. 1989 May;86(9):3311–3315. doi: 10.1073/pnas.86.9.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein Y., Ratnofsky S., Burakoff S. J., Herrmann S. H. Direct evidence for binding of CD8 to HLA class I antigens. J Exp Med. 1989 Jan 1;169(1):149–160. doi: 10.1084/jem.169.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saizawa K., Rojo J., Janeway C. A., Jr Evidence for a physical association of CD4 and the CD3:alpha:beta T-cell receptor. Nature. 1987 Jul 16;328(6127):260–263. doi: 10.1038/328260a0. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]