Abstract
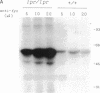
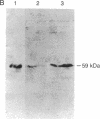
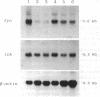
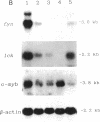
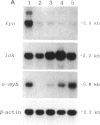
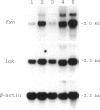
Overexpression of a src family gene, lck, has been associated with differentiation of the murine thymic lymphoma line LSTRA. Recent findings by several groups strongly suggest a functional role for the gene product p56lck protein-tyrosine kinase (PTK) in the activation of normal T cells. A single recessive gene, lpr or gld, induces a lymphoproliferative disorder concomitant with autoimmune disease in mice. In this study, a 10-fold elevated activity of PTK encoded by fyn, another src family gene, was demonstrated in CD4-CD8- T cells in mutant mice. The increased PTK activity was consistent with overexpression of fyn mRNA. The elevated fyn mRNA expression appeared to be a characteristic of CD4-CD8- T cells, since it was not observed in normal T cells at any stage of differentiation. The fact that fyn mRNA expression was markedly induced in normal T cells by mitogenic stimulation with anti-T3 epsilon antiserum supports the possibility that p59fyn PTK is a signal-generating molecule in T cells. Thus, our findings provide insight into the physiological role for a src gene family kinase in T-cell development and contribute to a better understanding of the molecular mechanisms of disease-inducing recessive genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Budd R. C., Cerottini J. C., MacDonald H. R. Cultured Lyt-2- L3T4- T lymphocytes from normal thymus or lpr mice express a broad spectrum of cytolytic activity. J Immunol. 1986 Dec 15;137(12):3734–3741. [PubMed] [Google Scholar]
- Budd R. C., Schreyer M., Miescher G. C., MacDonald H. R. T cell lineages in the thymus of lpr/lpr mice. Evidence for parallel pathways of normal and abnormal T cell development. J Immunol. 1987 Oct 1;139(7):2200–2210. [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Dumont F. J., Bedigian H. G., Fowlkes B. J., Morse H. C., 3rd Phenotypic, functional, and molecular genetic comparisons of the abnormal lymphoid cells of C3H-lpr/lpr and C3H-gld/gld mice. J Immunol. 1986 Jun 1;136(11):4075–4084. [PubMed] [Google Scholar]
- Davignon J. L., Cohen P. L., Eisenberg R. A. Rapid T cell receptor modulation accompanies lack of in vitro mitogenic responsiveness of double negative T cells to anti-CD3 monoclonal antibody in MRL/Mp-lpr mice. J Immunol. 1988 Sep 15;141(6):1848–1854. [PubMed] [Google Scholar]
- Earp H. S., Austin K. S., Gillespie G. Y., Buessow S. C., Davies A. A., Parker P. J. Characterization of distinct tyrosine-specific protein kinases in B and T lymphocytes. J Biol Chem. 1985 Apr 10;260(7):4351–4356. [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri K., Katagiri T., Eisenberg R. A., Ting J., Cohen P. L. Interleukin 2 responses of lpr and normal L3T4-/Lyt-2- T cells induced by TPA plus A23187. J Immunol. 1987 Jan 1;138(1):149–156. [PubMed] [Google Scholar]
- Katagiri K., Katagiri T., Yokota S., Eisenberg R. A., Cohen P. L., Ting J. P. Two different molecular pathways account for low IL-2 receptor and c-myc mRNA expression by lpr Lyt-2- L3T4- T cells. J Immunol. 1988 Sep 15;141(6):2145–2152. [PubMed] [Google Scholar]
- Katagiri T., Cohen P. L., Eisenberg R. A. The lpr gene causes an intrinsic T cell abnormality that is required for hyperproliferation. J Exp Med. 1988 Mar 1;167(3):741–751. doi: 10.1084/jem.167.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T., Tomiyama H., Kano K. Paul-Bunnell antigen in murine T cell differentiation: abnormal expression in MRL/Mp-lpr/lpr mice. J Immunol. 1986 Feb 1;136(3):913–919. [PubMed] [Google Scholar]
- Kawakami T., Kawakami Y., Aaronson S. A., Robbins K. C. Acquisition of transforming properties by FYN, a normal SRC-related human gene. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3870–3874. doi: 10.1073/pnas.85.11.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T., Pennington C. Y., Robbins K. C. Isolation and oncogenic potential of a novel human src-like gene. Mol Cell Biol. 1986 Dec;6(12):4195–4201. doi: 10.1128/mcb.6.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., O'Shea J. J., Luong H., Ross P., Bluestone J. A., Samelson L. E. T cell receptor tyrosine phosphorylation. Variable coupling for different activating ligands. J Biol Chem. 1987 Sep 15;262(26):12654–12659. [PubMed] [Google Scholar]
- Kotzin B. L., Babcock S. K., Herron L. R. Deletion of potentially self-reactive T cell receptor specificities in L3T4-, Lyt-2- T cells of lpr mice. J Exp Med. 1988 Dec 1;168(6):2221–2229. doi: 10.1084/jem.168.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. E., Giorgi J. V., Warner N. L. Flow cytometry analysis of T cells and continuous T-cell lines from autoimmune MRL/l mice. Nature. 1981 Jan 22;289(5795):298–300. doi: 10.1038/289298a0. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Lewis D. B., Wilson C. B., Gearn M. E., Krebs E. G., Perlmutter R. M. Regulation of pp56lck during T-cell activation: functional implications for the src-like protein tyrosine kinases. EMBO J. 1987 Sep;6(9):2727–2734. doi: 10.1002/j.1460-2075.1987.tb02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Melino M. R., Dumont F. J., Habbersett R. C., Hansen T. H. Expression of a bone marrow-associated Ly-6 determinant on the T cell population expanding in the lymph nodes of the autoimmune mouse strain MRL/Mp-lpr/lpr. J Immunol. 1983 Apr;130(4):1843–1847. [PubMed] [Google Scholar]
- Miescher G. C., Budd R. C., Lees R. K., MacDonald H. R. Abnormal expression of T cell receptor genes in Lyt-2- L3T4- lymphocytes of lpr mice: comparison with normal immature thymocytes. J Immunol. 1987 Mar 15;138(6):1959–1967. [PubMed] [Google Scholar]
- Morse H. C., 3rd, Davidson W. F., Yetter R. A., Murphy E. D., Roths J. B., Coffman R. L. Abnormalities induced by the mutant gene Ipr: expansion of a unique lymphocyte subset. J Immunol. 1982 Dec;129(6):2612–2615. [PubMed] [Google Scholar]
- Mountz J. D., Mushinski J. F., Mark G. E., Steinberg A. D. Oncogene expression in autoimmune mice. J Mol Cell Immunol. 1985;2(3):121–131. [PubMed] [Google Scholar]
- Nemazee D. A., Studer S., Steinmetz M., Dembić Z., Kiefer M. The lymphoproliferating cells of MRL-lpr/lpr mice are a polyclonal population that bear the T lymphocyte receptor for antigen. Eur J Immunol. 1985 Aug;15(8):760–764. doi: 10.1002/eji.1830150804. [DOI] [PubMed] [Google Scholar]
- Raveche E. S., Steinberg A. D., DeFranco A. L., Tjio J. H. Cell cycle analysis of lymphocyte activation in normal and autoimmune strains of mice. J Immunol. 1982 Sep;129(3):1219–1226. [PubMed] [Google Scholar]
- Reed J. C., Alpers J. D., Nowell P. C., Hoover R. G. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roths J. B., Murphy E. D., Eicher E. M. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med. 1984 Jan 1;159(1):1–20. doi: 10.1084/jem.159.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman E. M., Thom R. R., Casnellie J. E. Activation of a tyrosine protein kinase is an early event in the stimulation of T lymphocytes by interleukin-2. J Biol Chem. 1988 May 25;263(15):6956–6959. [PubMed] [Google Scholar]
- Samelson L. E., Davidson W. F., Morse H. C., 3rd, Klausner R. D. Abnormal tyrosine phosphorylation on T-cell receptor in lymphoproliferative disorders. Nature. 1986 Dec 18;324(6098):674–676. doi: 10.1038/324674a0. [DOI] [PubMed] [Google Scholar]
- Scholz W., Isakov N., Mally M. I., Theofilopoulos A. N., Altman A. Lpr T cell hyporesponsiveness to mitogens linked to deficient receptor-stimulated phosphoinositide hydrolysis. J Biol Chem. 1988 Mar 15;263(8):3626–3631. [PubMed] [Google Scholar]
- Semba K., Nishizawa M., Miyajima N., Yoshida M. C., Sukegawa J., Yamanashi Y., Sasaki M., Yamamoto T., Toyoshima K. yes-related protooncogene, syn, belongs to the protein-tyrosine kinase family. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5459–5463. doi: 10.1073/pnas.83.15.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A., Pyle R. H., Nagarkatti M., Nagarkatti P. S. Expression of the J11d marker on peripheral T lymphocytes of MRL-lpr/lpr mice. J Immunol. 1988 Aug 15;141(4):1120–1125. [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Hardy R. R., Seaman W. E. The proliferating cells in autoimmune MRL/lpr mice lack L3T4, an antigen on "helper" T cells that is involved in the response to class II major histocompatibility antigens. J Immunol. 1984 Jun;132(6):2686–2689. [PubMed] [Google Scholar]
- Yamanashi Y., Fukushige S., Semba K., Sukegawa J., Miyajima N., Matsubara K., Yamamoto T., Toyoshima K. The yes-related cellular gene lyn encodes a possible tyrosine kinase similar to p56lck. Mol Cell Biol. 1987 Jan;7(1):237–243. doi: 10.1128/mcb.7.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S., Yuan D., Katagiri T., Eisenberg R. A., Cohen P. L., Ting J. P. The expression and regulation of c-myb transcription in B6/lpr Lyt-2-, L3T4-T lymphocytes. J Immunol. 1987 Oct 15;139(8):2810–2817. [PubMed] [Google Scholar]