Abstract
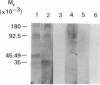
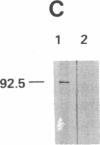
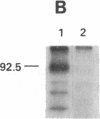
Cell surface receptors for the 86-kDa glycoprotein (gp86) of human cytomegalovirus (HCMV) were identified by using two monoclonal anti-idiotype antibodies that bear the internal image of gp86. These antibodies bound to cells permissive for HCMV infection by both ELISA and immunofluorescence assay and inhibited HCMV plaque formation in human embryonic lung (HEL) cells. Immunoblot analysis showed specific binding of both internal image anti-idiotype antibodies as well as gp86 to an HEL cell membrane protein with an approximate molecular mass of 92.5 kDa. In addition, immunoprecipitation of radiolabeled membrane and cell surface proteins from human foreskin tissue, human foreskin fibroblasts, or HEL cells showed specific binding of anti-idiotype antibody predominantly to the 92.5-kDa protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cai W. H., Gu B., Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988 Aug;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon D. J., Chamow S. M., Mordenti J., Marsters S. A., Gregory T., Mitsuya H., Byrn R. A., Lucas C., Wurm F. M., Groopman J. E. Designing CD4 immunoadhesins for AIDS therapy. Nature. 1989 Feb 9;337(6207):525–531. doi: 10.1038/337525a0. [DOI] [PubMed] [Google Scholar]
- Co M. S., Gaulton G. N., Fields B. N., Greene M. I. Isolation and biochemical characterization of the mammalian reovirus type 3 cell-surface receptor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1494–1498. doi: 10.1073/pnas.82.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Isola V. J., Kuhns J., Berman P. W., Eisenberg R. J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing ("native" gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986 Oct;60(1):157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erice A., Chou S., Biron K. K., Stanat S. C., Balfour H. H., Jr, Jordan M. C. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised patients. N Engl J Med. 1989 Feb 2;320(5):289–293. doi: 10.1056/NEJM198902023200505. [DOI] [PubMed] [Google Scholar]
- Gaulton G. N., Greene M. I. Idiotypic mimicry of biological receptors. Annu Rev Immunol. 1986;4:253–280. doi: 10.1146/annurev.iy.04.040186.001345. [DOI] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Gretch D. R., Kari B., Rasmussen L., Gehrz R. C., Stinski M. F. Identification and characterization of three distinct families of glycoprotein complexes in the envelopes of human cytomegalovirus. J Virol. 1988 Mar;62(3):875–881. doi: 10.1128/jvi.62.3.875-881.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Grundy J. E., McKeating J. A., Griffiths P. D. Cytomegalovirus strain AD169 binds beta 2 microglobulin in vitro after release from cells. J Gen Virol. 1987 Mar;68(Pt 3):777–784. doi: 10.1099/0022-1317-68-3-777. [DOI] [PubMed] [Google Scholar]
- Grundy J. E., McKeating J. A., Ward P. J., Sanderson A. R., Griffiths P. D. Beta 2 microglobulin enhances the infectivity of cytomegalovirus and when bound to the virus enables class I HLA molecules to be used as a virus receptor. J Gen Virol. 1987 Mar;68(Pt 3):793–803. doi: 10.1099/0022-1317-68-3-793. [DOI] [PubMed] [Google Scholar]
- Highlander S. L., Cai W. H., Person S., Levine M., Glorioso J. C. Monoclonal antibodies define a domain on herpes simplex virus glycoprotein B involved in virus penetration. J Virol. 1988 Jun;62(6):1881–1888. doi: 10.1128/jvi.62.6.1881-1888.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay S., Rasmussen L., Merigan T. C. Syngeneic monoclonal anti-idiotype antibodies that bear the internal image of a human cytomegalovirus neutralization epitope. J Immunol. 1988 Feb 1;140(3):944–948. [PubMed] [Google Scholar]
- Keller P. M., Davison A. J., Lowe R. S., Riemen M. W., Ellis R. W. Identification and sequence of the gene encoding gpIII, a major glycoprotein of varicella-zoster virus. Virology. 1987 Apr;157(2):526–533. doi: 10.1016/0042-6822(87)90295-9. [DOI] [PubMed] [Google Scholar]
- Krah D. L., Choppin P. W. Mice immunized with measles virus develop antibodies to a cell surface receptor for binding virus. J Virol. 1988 May;62(5):1565–1572. doi: 10.1128/jvi.62.5.1565-1572.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marriott S. J., Roeder D. J., Consigli R. A. Anti-idiotypic antibodies to a polyomavirus monoclonal antibody recognize cell surface components of mouse kidney cells and prevent polyomavirus infection. J Virol. 1987 Sep;61(9):2747–2753. doi: 10.1128/jvi.61.9.2747-2753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Miller N., Hutt-Fletcher L. M. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J Virol. 1988 Jul;62(7):2366–2372. doi: 10.1128/jvi.62.7.2366-2372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L. E., Nelson R. M., Kelsall D. C., Merigan T. C. Murine monoclonal antibody to a single protein neutralizes the infectivity of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):876–880. doi: 10.1073/pnas.81.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L., Kelsall D., Nelson R., Carney W., Hirsch M., Winston D., Preiksaitis J., Merigan T. C. Virus-specific IgG and IgM antibodies in normal and immunocompromised subjects infected with cytomegalovirus. J Infect Dis. 1982 Feb;145(2):191–199. doi: 10.1093/infdis/145.2.191. [DOI] [PubMed] [Google Scholar]
- Rasmussen L., Mullenax J., Nelson R., Merigan T. C. Viral polypeptides detected by a complement-dependent neutralizing murine monoclonal antibody to human cytomegalovirus. J Virol. 1985 Aug;55(2):274–280. doi: 10.1128/jvi.55.2.274-280.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L., Nelson M., Neff M., Merigan T. C., Jr Characterization of two different human cytomegalovirus glycoproteins which are targets for virus neutralizing antibody. Virology. 1988 Apr;163(2):308–318. doi: 10.1016/0042-6822(88)90271-1. [DOI] [PubMed] [Google Scholar]
- Stallcup K. C., Springer T. A., Mescher M. F. Characterization of an anti-H-2 monoclonal antibody and its use in large-scale antigen purification. J Immunol. 1981 Sep;127(3):923–930. [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]