Abstract
Pulmonary arterial hypertension (PAH), which is clinically characterized by a sustained elevation in mean pulmonary artery pressure leading to significant morbidity and mortality, is caused by intense remodeling of small pulmonary arteries by endothelial and smooth muscle proliferation. Genetic studies in familial PAH (FPAH) have revealed heterozygous germline mutations in the bone morphogenetic protein type II receptor (BMPR2), a receptor for the transforming growth factor (TGF)-β/BMP superfamily. In this study, we conducted mutation screening in the promoter region and the entire coding regions as well as the intron/exon boundaries of the BMPR2 gene in 20 Chinese patients with either idiopathic or FPAH. All novel detected mutations were excluded by their presence in a panel of 200 chromosomes from normal individuals. A novel mutation, G-669A, in the promoter sequence of the BMPR2 gene was identified in one patient with FPAH, and no exonic mutations were detected in the proband. This mutation abolished a potential specificity protein 3 (sp3) transcription factor-binding site, and a dual luciferase assay showed that the promoter carrying the −669A allele had significantly decreased transcriptional activity compared with −669G allele. Of the other 19 patients, three novel heterozygous exonic mutations were identified: a frame shift mutation with deletion of TG at the nucleotide position 608–609 in exon 5 (Leu203fsX15), a nonsense mutation at the nucleotide position 292 in exon 3 (Glu98X) and a missense single nucleotide substitution in exon 12 (Ser863Asn).
Keywords: bone morphogenetic protein receptor 2, pulmonary arterial hypertension, specificity protein 3
Introduction
Pulmonary arterial hypertension (PAH) is characterized by widespread obstruction and obliteration of the small pulmonary arteries with an estimated incidence of 1–2 per million cases per year, and a median survival from diagnosis of only 2.8 years.1 The arterial obstruction and obliteration cause a sustained elevation of pulmonary artery pressure and lead to right-heart failure with exercise limitation, palpitations, syncope and death.2 The development of PAH mainly affects small pulmonary arteries in which there is intense remodeling resulting in intimal thickening and medial hypertrophy because of an abnormal proliferation of smooth muscle and endothelial cells.3, 4
The most significant advance in understanding the pathogenesis of PAH has been the demonstration of germline mutations in the familial form of this disease that have been mapped to a single locus designated PPH1 (OMIM 178600) on chromosome 2q31–32.5 Subsequently, two independent groups identied heterozygous germline mutations in the gene encoding the bone morphogenetic protein type II receptor (BMPR2), a member of the transforming growth factor (TGF)-β superfamily of transmembrane serine/threonine kinase receptors, in patients with familial PAH (FPAH).6, 7 Although mutations in the BMPR2 gene (OMIM 600799) are present in more than approximately 70% of FPAH and up to 40% of patients with apparently sporadic idiopathic PAH (IPAH),8, 9, 10 no mutations in the promoter regions of the BMPR2 gene have been found to be associated with PAH. In this study, we reported, for the first time, a novel mutation G-669A in the promoter sequences and three novel exonic mutations of the BMPR2 in Chinese patients with PAH.
Materials and methods
Patients' data
Twenty unrelated patients with either IPAH or FPAH were enrolled. At the time of enrollment, the patients provided written informed consent according to a protocol approved by the institutional review board of the Cardiovascular Institute, Chinese Academy of Medical Sciences. Blood samples, detailed family histories and medical records were obtained. A review of complete medical records confirmed the diagnosis of IPAH or FPAH according to consensus standards.11 In brief, the diagnosis of IPAH required that a consultant with expertize in pulmonary vascular disease confirm the following: (1) mean pulmonary arterial pressure (PAP) >25 mm Hg, with a pulmonary capillary wedge pressure ≤15 mm Hg, both measured at rest by right heart catheterization and (2) the exclusion of other disorders known to cause pulmonary hypertension by clinical evaluation and objective tests, for example, ventilation and perfusion lung scans to exclude pulmonary embolism, contrast echocardiography and/or measurements of oxygen saturation during cardiac catheterization to exclude intracardiac shunting, and echocardiography and cardiac catheterization to exclude left heart disease.
Mutation detection
Genomic DNA was isolated from peripheral blood leukocytes with a standard salting-out protocol. The 2022 bp length sequences upstream of the initiation codon (nucleotides are numbered according to the DNA sequence, with the adenosine of the initiation codon assigned position +1) were divided into four fragments for amplification. PCR primers were designed to amplify the coding regions and the intron/exon boundaries of the BMPR2 gene in the 20 patients and a panel of 200 chromosomes from normal blood donors who were from the same ethnic/regional origin and age and gender matched with the patients. The sequences of each set of primers and PCR conditions are described in Table 1. The PCR products were purified and analyzed by direct sequencing using an ABI 3730 (Applied Biosystems, CA, USA). All novel detected mutations were excluded by their presence in a panel of 200 chromosomes from normal blood donors.
Table 1. The primer sequences used for PCR amplification.
| Exon | Sense primer | Antisense primer | Annealing Tm | Product length |
|---|---|---|---|---|
| Exon 1 | 5′-GGCTGTTTCTCCGCCGGTCTA-3′ | 5′-GGGTGGGAATATGGAAGTGGG-3′ | 58.9°C | 368 bp |
| Exon 2 | 5′-TCATGAACAGAAGAACGTCATT-3′ | 5′-CACAGTCATTTCAGGTAAGGA-3′ | 58.9°C | 374 bp |
| Exon 3 | 5′-GAAACTCCGCCTCAATAA-3′ | 5′-GAGACGGGATTTCACCAG-3′ | 51.2°C | 632 bp |
| Exon 4 | 5′-AGGAGCACATCTACTTGG-3′ | 5′-ATACTATTGAGGCTGGGT-3′ | 51.2°C | 483 bp |
| Exon 5 | 5′-TGGCTTTCATGCTATTCTGC-3′ | 5′-CCCCTTTTCATCACTTTCTTAT-3′ | 51.2°C | 588 bp |
| Exon 6 | 5′-AGGCTGGGTCTGGTAGGA-3′ | 5′-CCGGGTTCAAGCAATTCTC-3′ | 58.9°C | 652 bp |
| Exon 7 | 5′-TGGAATCCTAGCCTATTT-3′ | 5′-ACTCAGCTATCAAGCACC-3′ | 51.2°C | 452 bp |
| Exon 8 | 5′-GTATGTTCATTTCATGTTCAATAGTCC-3′ | 5′-AATTATCATTTCAAAGTACATCAGTGTG-3′ | 51.2°C | 315 bp |
| Exon 9 | 5′-ACATGGTTAGGGTCAAAT-3′ | 5′-GATGGTGCATAAGGAAAA-3′ | 51.2°C | 575 bp |
| Exon 10 | 5′-TTCCAAATGTGCCTGAAGGG-3′ | 5′-TGTGGCATTAGGCAACTCCAA-3′ | 51.2°C | 401 bp |
| Exon 11 | 5′-GAGCATGTTCCGTAATCC-3′ | 5′-TTGTTGGGTCTCAGTTTC-3′ | 51.2°C | 393 bp |
| Exon 12-1 | 5′-TGTACGTTTGGAAGAAAATG-3′ | 5′-ATTGTATTCATCTTGGCAACTC-3′ | 51.2°C | 913 bp |
| Exon 12-2 | 5′-GCAACTGGACAGCAGGACTT-3′ | 5′-AATAGTTATTTAAATGGCCCCA-3′ | 51.2°C | 779 bp |
| Exon 13 | 5′-CCAAGTTAAGATCGCTACCA-3′ | 5′-TGACAGGAGGATAAAGCAGT-3′ | 51.2°C | 670 bp |
Construction of luciferase reporter plasmids
Two 300 bp fragments, which carry the −669G and −669A allele, respectively, were amplified from the BMPR2 gene promoter region with specific primers (forward primer: 5′-GCAGCCTCTCACACCCACT and reverse primer: 5′-GGCAGCCCTAGTCGCATC). The PCR products which carry −669G and −669A allele were purified and cloned into the pGL3 basic vector fused with a luciferase reporter gene (Promega, Madison, WI, USA), generating two luciferase reporter constructs: pGL3-BMPR2-wild (carrying the −669G allele) and pGL3-BMPR2-mut (carrying the −669A allele). The two luciferase reporter constructs were sequenced to confirm the integrity and the presence of the desired mutation in the constructs. The pGL3 basic vector containing no promoter element was used as negative control and the pGL3 promoter vector fused with SV40 promoter element was used as positive control.
Cell culture
Human pulmonary artery endothelial cells (Cascade Biologics, Portland, OR, USA) were cultured in medium 200 (Cascade Biologics) supplemented with low serum growth supplement (Cascade Biologics). Human pulmonary artery smooth muscle cells (Cascade Biologics) were cultured in medium 231 (Cascade Biologics) supplemented with smooth muscle differentiation supplement (Cascade Biologics). The two cell cultures were supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, UT, USA), 100 μg/ml penicillin, 100 μg/ml streptomycin, and kept in an atmosphere of 95% air, 5% CO2 in a 37°C humidified incubator.
Transfection and luciferase assay
Human pulmonary artery endothelial cells and human pulmonary artery smooth muscle cells were cultured in 12-well plates. The cells were transfected transiently with the pGL3-BMPR2-wild and pGL3-BMPR2-mut vectors using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After 48 h, cell lysates were prepared with the Dual-Luciferase reporter assay system (Promega, Madison, WI, USA), and both Firefly and Renilla luciferase activities were measured in a Veritas Microplate Luminometer (Turner, Sunnyvale, CA, USA). The transfection efficiency was normalized according to the Renilla luciferase activity. Each transfection was carried out in quintuplicate and repeated three times.
Statistical analysis
All data were expressed as mean±SD. Differences between groups were examined for statistical significance using Student's t-test. A P-value <0.05 denoted the presence of a statistically significant difference.
Results
Genetic findings
To detect variants presenting in the promoter region of the BMPR2 gene that potentially regulate the level of gene transcription, the 2022 bp length sequence upstream of the initiation codon was amplified in 20 IPAH or FPAH patients. Direct sequencing of the PCR products revealed that one patient with FPAH carried a novel heterozygous substitution mutation G-669A in the promoter region of the BMPR2 gene and no other mutations were found in any of the 13 exons in the proband (Figures 1 and 2). The other 19 PAH patients and the panel of 200 chromosomes from normal individuals were found not to harbor this mutation. Of the other 19 patients, three novel heterozygous exonic mutations were identified: a frame shift mutation with deletion of TG at the nucleotide position 608–609 in exon 5 (Leu203fsX15), a nonsense mutation at the nucleotide position 292 in exon 3 (Glu98X) and a missense single nucleotide substitution in exon 12 (Ser863Asn). Moreover, only one mutation, Cys66Arg, in exon 2 was found to be one of the more than 140 previously reported mutations in this study (Table 2).
Figure 1.
The pedigree map of the familiar pulmonary arterial hypertension family from China.
Figure 2.
Sequence analysis of the heterozygous BMPR2 G-669A mutation in the proband. (a) and (b) indicate the forward and reverse sequence analyses of the BMPR2 gene promoter region in a healthy control individual, respectively. The G-669A mutation present in the proband (II-2) is shown in (c) and (d), which indicate the BMPR2 forward and reverse sequence analyses.
Table 2. BMPR2 mutations in Chinese PAH patientsa.
| Sex | Age (years) | Location | Domain | Mutation type | Nucleotide change | Amino-acid change | IPAH or FPAH |
|---|---|---|---|---|---|---|---|
| Female | 43 | Exon 2 | ECD | Missense | c.196T>C | Cys66Arg | IPAH |
| Female | 34 | Exon 3 | ECD | Nonsense | c.292G>T | Glu98Xb | IPAH |
| Female | 20 | Exon 5 | Frameshift | c.608-609delTG | Leu203fsX15b | IPAH | |
| Male | 34 | Exon 12 | CD | Missense | c.2588G>A | Ser863Asnb | FPAH |
CD, cytoplasmic domain; ECD, extracellular domain; FPAH, familial pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension.
GenBank reference sequence and version number for BMPR2: NM_001204.6; numbering is based on +1 as A of the ATG initiation codon.
Novel mutations reported in this study.
Clinical findings
Figures 1 and 2 show the pedigree of the proband (II-2) carrying the G-669A mutation. The proband was a 38-year-old female who was diagnosed at the age of 36 with dyspnea on exertion, hemoptysis and severe PAH. Echocardiography showed an enlarged right atrium (RA) and right ventricle (RV) with regurgitation through the tricuspid valve. Right heart catheterization revealed elevated PAP: 74/30 (46) mm Hg. The proband's brother and father had similar clinical manifestations and both died of IPAH in their twenties (Figure 1).
BMPR2 promoter carrying the -669A allele has decreased transcription activity compared with the −669G allele
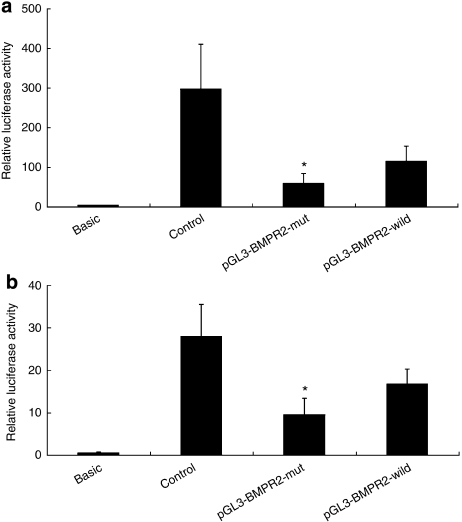
To verify the effect of the variants −669A and −669G on the transcriptional activity of the BMPR2 gene, two luciferase reporter vectors, which contain −669A allele or −669G allele, were constructed. Human pulmonary artery endothelial cells and human pulmonary artery smooth muscle cells were transiently transfected with the two vectors and luciferase activities were measured. The results showed that the BMPR2 promoter carrying the −669A allele showed significantly decreased transcriptional activity compared with that carrying the −669G allele in human pulmonary artery endothelial cells (Figure 3a) and in human pulmonary artery smooth muscle cells (Figure 3b).
Figure 3.
Transcriptional activity analysis of the BMPR2 promoter containing the −669G and −669A alleles. Two luciferase reporter constructs (pGL3-BMPR2-wild and pGL3-BMPR2-mut) containing 300 bp of the BMPR2 promoter sequence, which carry −669G and −669A alleles, respectively were introduced into human pulmonary artery endothelial cells and human pulmonary artery smooth muscle cells for transient expression assays. After 48 h, cells were lysed and luciferase activities were assayed. Firefly luciferase activities expressed were normalized on the basis of Renilla luciferase activity encoded by the cotransfected control plasmid, pRL-CMV. The results showed that the luciferase activity of the BMPR2 promoter containing the −669A allele (mutant) was significantly decreased compared with the −669G allele (wild type) in endothelial cells (a) and in smooth muscle cells (b). Each transfection was carried out in quintuplicate and repeated three times. Data shown are the means±SD., n=5 (*compared with the relative luciferase activity of −669G allele in endothelial cells, P=0.025 and in smooth muscle cells, P=0.015). The pGL3 basic vector containing no promoter element was used as negative control and the pGL3 promoter vector fused with SV40 promoter element was used as positive control.
Identification of putative transcription factor-binding sites affected by the −669G and −669A alleles of BMPR2 gene
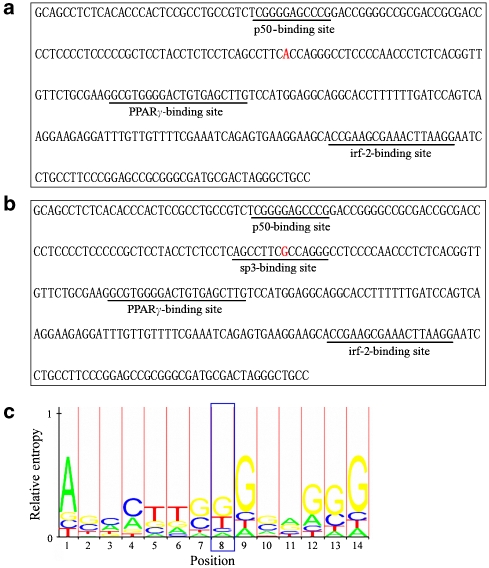
Using the MAPPER Search Engine,12 we identified a putative transcription factor-binding site affected by the −669G and −669A alleles. Figure 4b shows that the BMPR2 promoter carrying the −669G contains a consensus-binding site for specificity protein 3 (sp3) transcription factor. G-669A mutation abolishes the sp3-binding site (Figure 4a). This change (−669G>A) altered the highly conserved G base in the sp3-binding site (Figure 4c).
Figure 4.
Identification of putative transcription factor-binding sites affected by −669G and −669A alleles of BMPR2 gene. Using the MAPPER Search Engine (http://mapper.chip.org/), we identified the putative transcription factor-binding sites affected by −669G and −669A alleles. (b) The BMPR2 promoter carrying the −669G allele contains a consensus-binding site for sp3 transcription factor. After the G was changed to A, the sp3-binding site was abolished (a). This change (-669G>A) altered the highly conserved G base in the sp3-binding site (c). The sequence motif of the profile representing the binding site is shown.
Discussion
This is the first report that a mutation in the promoter sequence of the BMPR2 gene is associated with FPAH. In earlier studies, extensive analyses of the BMPR2 gene have been conducted in patients with FPAH and IPAH from a wide range of ethnic groups. Recently, two novel missense mutations of BMPR2 gene have been identified in two Chinese families with PAH.13, 14 Mutations of BMPR2 gene have been detected in up to 70% of recognized FPAH cases, whereas in IPAH, the reported mutation detection rate ranges from 11 to 40%.8, 9, 10 All the defects of BMPR2 gene identified in patients, which include all major mutation classes, with single nucleotide substitutions resulting in nonsense,6, 7, 8, 9, 10, 15, 16, 17, 18, 19, 20 missense,6, 7, 8, 9, 10, 15, 16, 17, 18, 21, 22, 23, 24, 25 or splice site mutation,9, 15, 17, 21 and small insertions or deletions6, 7, 8, 9, 15, 16, 17, 19, 21, 22, 26, 27, 28, 29 are observed in exons, introns or intron/exon boundaries. No mutations were reported to be found in the promoter region of BMPR2 gene in patients with FPAH or IPAH previously. In this study, a novel heterozygous substitution mutation, G-669A, in the promoter sequence of BMPR2 gene was identified in a Chinese patient with FPAH.
The TGF-β superfamily of ligands and receptors may be divided into two principal classes, termed the TGF-βs and BMPs, based on sequence comparison and Smad-dependent intracellular signaling activity. BMPs are the largest group of cytokines within the TGF-β superfamily and are involved in the regulation of proliferation, differentiation and apoptosis.30 Signaling by BMP receptors involves heterodimerization of two transmembrane serine/threonine kinases: the constitutively active type II receptor, BMPR2, and a corresponding type I receptor, BMPR1A or BMPR1B. The most comprehensively investigated mediators of TGF-β signal transduction are the Smad family and can be divided into three classes: receptor (R-) Smads, collaborating (Co-) Smads and inhibitory (I-) Smads. Of the three Smads, R-Smads 1/5 are specific to BMP signal flow. BMPR2 binds the specic ligands to initiate and propagate intracellular signal by activating R-Smads 1/5. Activated R-Smads form a heteromeric complex with Co-Smad 4, translocating to the nucleus, to control the transcriptional regulation of target genes.31, 32 R-Smads 2/3 are the important mediators of TGF-β signaling, their activation can lead to abnormal proliferative responses to TGF-β and R-Smads 2/3 have been proved to functionally antagonize R-Smads 1/5 signaling.33, 34, 35 Earlier studies have shown that BMPR2 mutations lead to a loss of signaling through Smad1/5 in cells and animal models.25, 36 Thus, failure of BMP signaling through Smad1/5 in the vasculature can increase TGF-β/Smad2/3 signaling, which highlights the potential importance of a loss of Smad1/5 signaling as cause of pulmonary vascular remodeling in patients with genetic PAH. In our study, the mutation −669A in the promoter sequence of BMPR2 gene showed decreased transcriptional activity and may downregulate the expression of BMPR2 and weaken Smad1/5 signaling, similarly to BMPR2 coding region mutations.
We performed a computer analysis of the 300 bp promoter sequence that carries the −669G allele using CpG Island Searcher (http://www.uscnorris.com/cpgislands2/cpg.aspx). The result showed that this promoter region is a potential CpG Island. Methylation of CpG sites within the promoters of genes can lead to their silencing, and a feature found in a number of human cancers (for example, the silencing of tumor suppressor genes). In contrast, the demethylation of CpG sites has been associated with the overexpression of oncogenes within cancer cells. The −669G allele changed to −669A allele in the 300 bp promoter region of BMPR2 gene might have abolished a potential CpG site and resulted in its demethylation. This demethylation might cause an upregulation of the transcriptional activity of the BMPR2 gene. However, in our study, we found that the transcriptional activity of the BMPR2 promoter sequence carrying −669A allele decreased significantly compared with −669G allele in luciferase activity assay experiments; therefore, we deduced that this potential CpG site might not be functional.
Searching in the MAPPER transcription factor database,12 a potential sp3 transcription factor-binding site was identified in the BMPR2 promoter sequence carrying the −669G allele, which is abolished by the G-669A mutation. This was of interest because sp3 is a well-known transcription factor with important regulatory effects on various cellular and viral promoters. The sp family of transcription factors binds and acts through GC boxes to regulate gene expression and is recognizable by a DNA-binding domain formed from three conserved Cys2His2 zinc ngers.37 Within this family, sp3 and sp1 are ubiquitously expressed in mammalian cells, and sp3 is structurally similar to sp1, with similar affinities for the sp1-binding site, and participates in regulating the expression of genes involved in almost all cellular processes.38 Sp3 has been shown to act as a transcriptional activator similar to sp1 in many studies.39, 40, 41 Our results showed that the −669A mutant decreased luciferase transcriptional activity and had an abolished sp3 transcription factor-binding site. This suggests that sp3 may play an important role in the transcriptional regulation of BMPR2 gene.
The BMPR2 gene consists of 13 exons. The mature protein harbors four discrete functional domains, including an extracellular ligand-binding domain encoded by exons 1–3, a transmembrane domain generated by exon 4, a serine/threonine kinase domain from exon 5–11 and a very large intracellular C-terminal domain of unknown function from exons 12 and 13.16 This large C-terminal domain is present only in BMPR2 within the TGF-β receptor superfamily. More than 140 distinct mutations of BMPR2 gene have been identified in patients with PAH from a wide range of ethnic groups.8 In this study, we identified three novel exonic mutations of the BMPR2 gene in the Chinese PAH patients (Table 2), including a frame shift mutation in exon 5 (Leu203fsX15), a nonsense mutation in exon 3 (Glu98X) and a missense mutation in exon 12 (Ser863Asn). These disease-causing mutations are predicted to affect different domains of the BMPR2 protein and mutations within different regions may vary in their impact on disease initiation and progression. Only one mutation (Cys66Arg) of more than 140 mutations reported earlier has been found in Chinese PAH patients in this study. The ethnic group difference may be a potential explanation.
It is well known that nonsense, missense, splice site mutations and small insertions or deletions in the genes can lead to the expression of defective protein products, which are often responsible for a hereditary disease. In our study, we have reported four novel mutations of the BMPR2 gene in Chinese patients with PAH. This posed some interesting questions, such as whether the disease can be caused by the reduced level of mRNA expression and further explorations in the future may provide us a complete picture of the role of BMPR2 in the etiology of PAH
Acknowledgments
This study was supported by the National Natural Science Foundation of China (nos. 30570796 and 30600239).
References
- Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- Cool CD, Stewart JS, Werahera P, et al. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol. 1999;155:411–419. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols WC, Koller DL, Slovis B, et al. Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31-32. Nat Genet. 1997;15:277–280. doi: 10.1038/ng0397-277. [DOI] [PubMed] [Google Scholar]
- Deng Z, Morse JH, Slager SL, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane KB, Machado RD, Pauciulo MW, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- Machado RD, Aldred MA, James V, et al. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat. 2006;27:121–132. doi: 10.1002/humu.20285. [DOI] [PubMed] [Google Scholar]
- Morisaki H, Nakanishi N, Kyotani S, Takashima A, Tomoike H, Morisaki T. BMPR2 mutations found in Japanese patients with familial and sporadic primary pulmonary hypertension. Hum Mutat. 2004;23:632. doi: 10.1002/humu.9251. [DOI] [PubMed] [Google Scholar]
- Thomson JR, Machado RD, Pauciulo MW, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet. 2000;37:741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- Marinescu VD, Kohane IS, Riva A. MAPPER: a search engine for the computational identification of putative transcription factor binding sites in multiple genomes. BMC Bioinformatics. 2005;6:79. doi: 10.1186/1471-2105-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LJ, Zhou AQ, Huang MR, et al. A novel mutation in the BMPR2 gene in familial pulmonary arterial hypertension. Chin Med J (Engl) 2008;121:399–404. [PubMed] [Google Scholar]
- Zhicheng J, Lihe L, Zhiyan H, et al. Bone morphogenetic protein receptor-II mutation Arg491Trp causes malignant phenotype of familial primary pulmonary hypertension. Biochem Biophys Res Commun. 2004;315:1033–1038. doi: 10.1016/j.bbrc.2004.01.158. [DOI] [PubMed] [Google Scholar]
- Koehler R, Grunig E, Pauciulo MW, et al. Low frequency of BMPR2 mutations in a German cohort of patients with sporadic idiopathic pulmonary arterial hypertension. J Med Genet. 2004;41:e127. doi: 10.1136/jmg.2004.023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado RD, Pauciulo MW, Thomson JR, et al. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet. 2001;68:92–102. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CG, Glissmeyer EW, Havlena GT, et al. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation. 2006;113:2509–2515. doi: 10.1161/CIRCULATIONAHA.105.601930. [DOI] [PubMed] [Google Scholar]
- Humbert M, Deng Z, Simonneau G, et al. BMPR2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Respir J. 2002;20:518–523. doi: 10.1183/09031936.02.01762002. [DOI] [PubMed] [Google Scholar]
- Harrison RE, Berger R, Haworth SG, et al. Transforming growth factor-beta receptor mutations and pulmonary arterial hypertension in childhood. Circulation. 2005;111:435–441. doi: 10.1161/01.CIR.0000153798.78540.87. [DOI] [PubMed] [Google Scholar]
- Uehara R, Suzuki H, Kurokawa N, et al. Novel nonsense mutation of the BMPR-II gene in a Japanese patient with familial primary pulmonary hypertension. Pediatr Int. 2002;44:433–435. doi: 10.1046/j.1442-200x.2002.01567.x. [DOI] [PubMed] [Google Scholar]
- Cogan JD, Vnencak-Jones CL, Phillips JA, III, et al. Gross BMPR2 gene rearrangements constitute a new cause for primary pulmonary hypertension. Genet Med. 2005;7:169–174. doi: 10.1097/01.gim.0000156525.09595.e9. [DOI] [PubMed] [Google Scholar]
- Sankelo M, Flanagan JA, Machado R, et al. BMPR2 mutations have short lifetime expectancy in primary pulmonary hypertension. Hum Mutat. 2005;26:119–124. doi: 10.1002/humu.20200. [DOI] [PubMed] [Google Scholar]
- Roberts KE, McElroy JJ, Wong WP, et al. BMPR2 mutations in pulmonary arterial hypertension with congenital heart disease. Eur Respir J. 2004;24:371–374. doi: 10.1183/09031936.04.00018604. [DOI] [PubMed] [Google Scholar]
- Morrell NW, Yang X, Upton PD, et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation. 2001;104:790–795. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- Rudarakanchana N, Flanagan JA, Chen H, et al. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet. 2002;11:1517–1525. doi: 10.1093/hmg/11.13.1517. [DOI] [PubMed] [Google Scholar]
- Aldred MA, Vijayakrishnan J, James V, et al. BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Hum Mutat. 2006;27:212–213. doi: 10.1002/humu.9398. [DOI] [PubMed] [Google Scholar]
- Trembath RC, Thomson JR, Machado RD, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2001;345:325–334. doi: 10.1056/NEJM200108023450503. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Hirota H, Yoshida M, et al. Novel insertional mutation in the bone morphogenetic protein receptor type II associated with sporadic primary pulmonary hypertension. Circ J. 2004;68:592–594. doi: 10.1253/circj.68.592. [DOI] [PubMed] [Google Scholar]
- Abramowicz MJ, Van Haecke P, Demedts M, Delcroix M. Primary pulmonary hypertension after amfepramone (diethylpropion) with BMPR2 mutation. Eur Respir J. 2003;22:560–562. doi: 10.1183/09031936.03.00095303. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Thorikay M, Kowanetz M, et al. Elucidation of Smad requirement in transforming growth factor-beta type I receptor-induced responses. J Biol Chem. 2003;278:3751–3761. doi: 10.1074/jbc.M208258200. [DOI] [PubMed] [Google Scholar]
- Jeffery TK, Upton PD, Trembath RC, Morrell NW. BMP4 inhibits proliferation and promotes myocyte differentiation of lung fibroblasts via Smad1 and JNK pathways. Am J Physiol Lung Cell Mol Physiol. 2005;288:L370–L378. doi: 10.1152/ajplung.00242.2004. [DOI] [PubMed] [Google Scholar]
- Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280:24443–24450. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- Cawley S, Bekiranov S, Ng HH, et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell. 2004;116:499–509. doi: 10.1016/s0092-8674(04)00127-8. [DOI] [PubMed] [Google Scholar]
- Udvadia AJ, Templeton DJ, Horowitz JM. Functional interactions between the retinoblastoma (Rb) protein and Sp-family members: superactivation by Rb requires amino acids necessary for growth suppression. Proc Natl Acad Sci USA. 1995;92:3953–3957. doi: 10.1073/pnas.92.9.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihn H, Trojanowska M. Sp3 is a transcriptional activator of the human alpha2(I) collagen gene. Nucleic Acids Res. 1997;25:3712–3717. doi: 10.1093/nar/25.18.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chang LS. The human POLD1 gene. Identification of an upstream activator sequence, activation by Sp1 and Sp3, and cell cycle regulation. J Biol Chem. 1997;272:4869–4882. [PubMed] [Google Scholar]