Abstract
Scorpion venom is a complex mixture of salts, small molecules, peptides, and proteins. Scorpions employ this valuable tool in several sophisticated ways for subduing prey, deterring predators, and possibly during mating. Here, a subtle but clever strategy of venom utilization by scorpions is reported. Scorpions secrete a small quantity of transparent venom when initially stimulated that we propose to name prevenom. If secretion continues, a cloudy and dense venom that is white in color is subsequently released. The prevenom contains a combination of high K+ salt and several peptides including some that block rectifying K+ channels and elicit significant pain and toxicity because of a massive local depolarization. The presence of high extracellular K+ in the prevenom can depolarize cells and also decrease the local electrochemical gradient making it more difficult to reestablish the resting potential. When this positive change to the K+ equilibrium potential is combined with the blockage of rectifying K+ channels, this further delays the recovery of the resting potential, causing a prolonged effect. We propose that the prevenom of scorpions is used as a highly efficacious predator deterrent and for immobilizing small prey while conserving metabolically expensive venom until a certain level of stimuli is reached, after which the venom is secreted.
Like the snails of the genus Conus (1, 2), scorpions have developed efficient venoms. They occupy temperate and tropical habitats of the world. They are especially well adapted to survive in extreme thermal environments, sometimes constituting a major portion of the total animal biomass in these environments (3). Scorpions are considered among the successful inhabitants of earth (4). Although numerous factors contribute to the success of scorpions, perhaps the ability to produce and deliver a highly toxic secretion, the venom, is an important determinant in this success. Venom is used by scorpions to subdue prey and also as a defense against predators. Although all scorpion species are known to possess venom, only about 25–50 of 1,250 known species are considered medically important (5–7).
The scorpion venom, a unique weapon, is a secretion composed of water, salts, small molecules, peptides, and proteins (8). The venoms of several scorpion species have been well characterized, and peptides possess the majority of the biological activity (9). In the venom mixture, there are peptides that are specialized against vertebrates, invertebrates, or active against both. Members of all three groups are well characterized and include peptides that target all of the major ion channel types such as Na+, K+, Cl−, Ca2+, and ryanodine-sensitive Ca2+ channels (9–12). The devastating potency of the venom is caused by its ability to target multiple types of ion channels simultaneously, resulting in a massive and recurring depolarization that disables or kills the prey or predator.
Production and storage of protein-rich venom is undoubtedly an expensive metabolic investment, especially for species adapted to survive in extreme ecosystems on scarce resources. Other than the antimicrobial peptides, almost all neurotoxins in the venom are reported to be highly folded, disulfide-bridged molecules (13). Low yields of expression of these peptides in recombinant systems also hint at the unique and difficult folding and storage requirements (14). However, used sparingly, venom is an excellent tool for both offense and defense. A study on the sting use of Parabuthus species is a good example of the regulation of sting use according to prey size, demonstrating the conservative use of venom (15). The conservative use of venom by many species of scorpions suggests that venom secretion is also regulated.
Parabuthus transvaalicus (Purcell, 1899) is a very large and medically important scorpion species (16–18). In our studies with this scorpion, we observed that the first droplet of venom that is secreted has different physical properties than the rest of the venom. We refer to the first droplet as “prevenom.” Separate collection of prevenom from venom has enabled us to study its properties compared with venom. This phenomenon was observed by Yahel-Niv and Zlotkin by using Leiurus quinquestriatus (Ehrenberg, 1828; see ref. 19 and references therein). These authors reported that the appearance of venom changes from transparent to opalescent and to viscous secretions in successive stings. Their characterization concluded that each one of the three forms has different protein bands, and that these coincide with the depletion of venom from the venom glands of scorpions. However, the transparent venom was not recognized as a pharmacologically different secretion by these authors. Here, we report that the prevenom is a particular type of venom with unique properties and with a different molecular mechanism of action than that of the venom. Its use, on the one hand, helps conserve the more valuable venom, whereas on the other hand, it provides superb toxicity.
Materials and Methods
Animal Rearing and Venom Collection.
Scorpions (P. transvaalicus) were kept individually in plastic containers under 12-h light/12- h dark cycle at 28°C constant temperature. For venom collection, 1.5-ml microfuge tubes are covered with a piece of parafilm, and scorpions are induced to sting through the parafilm. Prevenom and venom are collected separately. The samples are frozen at −20°C until use.
Chemical and Biochemical Analysis.
Ionic salt content of prevenom and venom are determined at University of California, Davis, Division of Agriculture and Natural Resources by using atomic absorption spectrometry. Protein concentration was determined with Bradford assay by using BSA as the standard. A Michrom Bioresources (Auburn, CA) Magic 2002 microbore HPLC system equipped with a 1-mm inside diameter C18 reverse-phase column and a 5-μm peptide trap is used for HPLC analysis, as described (20).
Bioassays.
Biological activity of the prevenom and the venom are assessed by injecting insects and mice. Insects Trichoplusia ni (cabbage looper) and Sarcophaga bullata (flesh fly) were obtained from Carolina Biological Supply and raised in our laboratory. Swiss–Webster male mice were obtained from Charles River Breeding Laboratories and housed at the University of California, Davis, Animal Housing Facility. Effects of salt and prevenom or venom were quantified by injecting insect larvae with various concentrations of salt or venom protein. Lethal dose for prevenom and venom were determined by probit analysis with POLO software (21). LD99 for mice was determined as described (20). Test animals were kept individually and observed over a period of one day. Pain was quantified by the number of times mice licked their feet within a 10-min postinjection period. Animal protocols were approved by the University of California, Davis.
Mass Spectrometry.
Mass spectrometry was partly done as described previously by using a Biflex Bruker matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) instrument (20). A quadrapole orthogonal TOF mass spectrometer with QqT geometry (QSTAR, Applied Biosystems) was also used. The MS was controlled by ANALYST-QS V.1.0 software with Service Pack 3 installed and equipped with a nano-spray ionization source (Protana, Odense, Denmark). Voltage on the Protana capillary tips was set to 900 V. External two-point calibration was performed by using the singly charged ions of CsI and a peptide with nominal mass of 828.5. Mass accuracy was typically better than 10 ppm, and resolution was typically better than 7,000 full-width half maximum definition. The flow rate of the nanospray source was set to ≈25–50 nl/min. Nitrogen was used as the curtain gas at 25 arbitrary units. The orifice voltage was set at 80 V for all samples, the focusing ring was set to 250 V, and the skimmer was kept at 30 V. The TOF analyzer was set to acquire spectra at a rate of 7 kHz over the mass range 100–2,000 Da. The results are the average of 3–10 consecutive spectra. MassLynx (Micromass, Manchester, U.K.) software was used for data processing and analysis. All components of both the venom and the prevenom were analyzed by both MALDI-TOF and electrospray mass spectrometry.
Membrane Depolarization Assay.
Membrane depolarization assays were conducted by using ratio fluorescent imaging of differentiated C2C12 cells (mouse skeletal muscle cells, American Type Culture Collection) as described (22). Cells were allowed to differentiate 6–7 days until long myotubes formed and occasional spontaneous contractions were seen in the culture. Depolarization was observed as an increase in the fura-2 fluorescence ratio intensity because of depolarization-induced Ca2+ transient. Application of high doses of venom caused death in cells, as observed by a steady high signal without any further response to subsequent applications of high K+, whereas prevenom at the same quantity did not cause lethality. Dead cells resulting from high concentrations of venom application were removed from further analysis. The data are presented as the ratio of the emissions obtained at 340 and 380 nm (340/380).
Statistical Analysis.
Data are subjected to principle components analysis (PCA) by using MATLAB (Mathworks, Natick, MA) software package (23). PCA was used because it has superior filtering of noise and averaging capability than plain averaging. The PCA signal that represents Ca2+ transient of treatments is the sum of squares of the latent variables that capture 95% of the variability in region of interest. Peak area under the signal was calculated and plotted for each dose of prevenom and venom.
Results
Prevenom Is Secreted Before Venom.
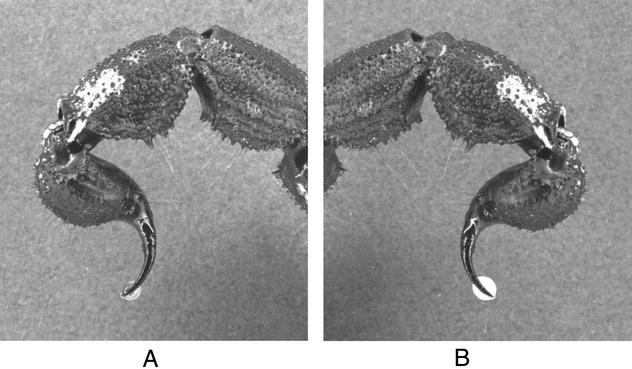
When stimulated, scorpions first secrete a “transparent” version of their venom, which we call the prevenom (Fig. 1A). If threat or prey mobility continues, they secrete an “opaque” venom, which is white in color and loaded with toxins at ≈53–85 mg/ml protein concentration (Fig. 1B). Prevenom constitutes ≈5% of the secreted venom in volume (Table 1). In some cases where the individual scorpion is aggressive, it is difficult to obtain prevenom separately from venom. By the time these scorpions are restrained, they start secreting the venom. However, even in these cases, it is possible to observe prevenom/venom mixture during the milking process, during which the clear and cloudy portions mix slowly on the side of the microfuge tube. Similar behavior has been observed in several species in our laboratory, including Androctonus australis (Linnaeus, 1758) and Uroctonus mordax (Thorell, 1876).
Figure 1.
(A) Prevenom is secreted first when a scorpion is threatened. This is a small and transparent droplet. (B) Venom follows the prevenom. Venom is highly potent and rich in peptides and proteins.
Table 1.
Comparative properties of prevenom and venom
| Protein mg/ml, n = 8 | Potassium salt, mM | Volume secreted, μl | PD50 insect, mg protein/100 mg larvae (μl venom/100 mg larvae) | LD99 mouse mg protein/20-g mouse (μl venom/20-g mouse) | Pain response (foot licking for 10 min after injection) | |
|---|---|---|---|---|---|---|
| Prevenom | 7.2–14.3 | 80.2 ± 4.5 | 1.2 ± 0.6 | 0.00028 (0.028) | 0.0041 (0.5) | 32 ± 2.6 |
| Venom | 53.4–85.2 | 5.4 ± 0.4 | 21 ± 5.2 | 0.00081 (0.016) | 0.0048 (0.1) | 9.3 ± 2.0 |
Prevenom and Venom Are Chemically Different.
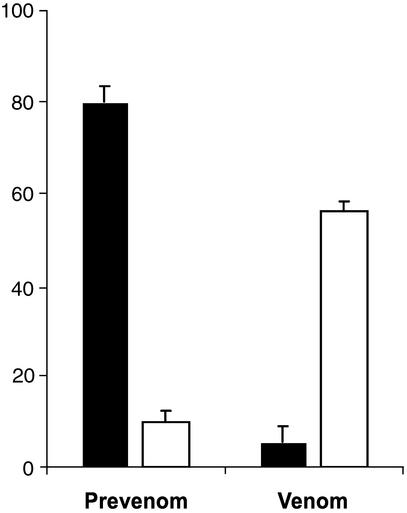
Our initial attempts to characterize the prevenom by MALDI-TOF MS failed because of the high concentration of K+ ions. The K+ salt content of prevenom and venom was quantified by using AAS (Fig. 2). The prevenom of P. transvaalicus has an unusually high K+ salt concentration.
Figure 2.
Prevenom (left) contains high K+ salt concentration (mM, black bar) and low quantities of protein (mg/ml, white bar). In contrast, venom (right) contains very high quantity of protein (mg/ml) and a physiological concentration of K+ salt (mM). Variation is expressed as SD among three individuals.
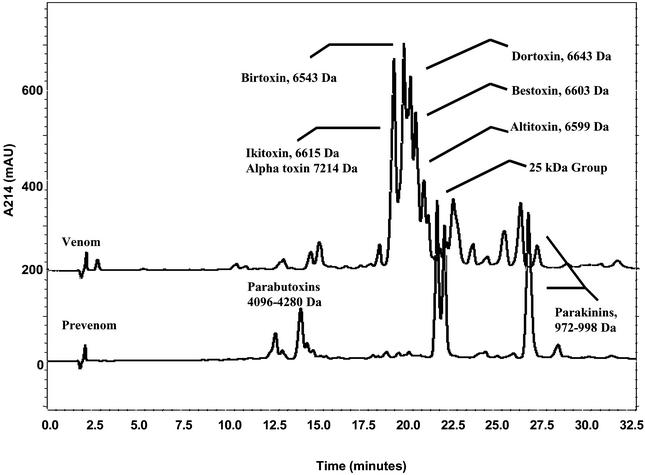
Comparison of the peptidic profiles of prevenom and venom by RP-HPLC reveals significant differences between the two types of venom (Fig. 3). The venom of P. transvaalicus contains at least 100 peptides as determined by MALDI-TOF MS (20). In contrast, three major groups of peptides were detected in the prevenom by using MS. The first group is most abundant, with peptides in the mass range of 700–1,200 Da, and a 986.6 Da (M+H)+ principle peptide component. The second group is thought to be composed of members of a previously discovered group of K+ channel blockers, including peptides similar to parabutoxin 1 (4,096 Da) and parabutoxin 2 (4,108 Da). These peptides are known to block the rectifying Kv1.1 K+ channels (11). In this group, the most intensive ion detected was the 4,090.6-Da peak similar to parabutoxin 1, along with its K+ adduct, the 4,128-Da peak. Parabutoxin 2-like peptide was also detected together with its K+ adduct, giving a mass of 4,141.5 Da. The third group is composed of several proteins of 25,000 Da, the major component being 25,208 Da, and several other components including 25,224 Da, 25,238 Da, and 25,267 Da species. All of these components are also present in the venom, albeit in different proportions.
Figure 3.
RP-HPLC profiles of prevenom and venom. Prevenom and venom are run on a C18 microbore column, and the UV trace at 214 nm is followed. Major components are identified by liquid chromatography (LC)-MS. Both prevenom and venom contain the 0.9- to 1.2-kDa group, the 3- to 4-kDa group, and the 25-kDa group. However, most of the protein in venom corresponds to the 6- to 7-kDa peptide group. Moreover, the three protein groups in prevenom are major species because of the lack of a 6- to 7-kDa group.
Prevenom Is Active Against both Insects and Mammals.
Determination of dose–response (i.e., paralysis) curves for K+ and Na+ alone by injecting flesh fly and cabbage looper larvae shows that increased extracellular Na+ has no visible effect on paralysis, being lethal only at very high doses (>1,000 mM), whereas K+ immediately paralyzes the insects with an EC50 of 43 mM (K+ injected in 3 μl per 150 mg of larvae) for cabbage looper (see Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org). This finding is in agreement with the physiological role of K+ ion and indicates that high K+ alone may contribute to the transient immobilization of the prey. The toxicity of the prevenom and venom were compared by bioassays using cabbage looper (Table 1).
The paralytic activity of prevenom to cabbage looper is about 2.8-fold higher on a protein basis than that of the venom, although it is 1.7-fold lower on a volumetric basis (per microliter of venom injected). Both prevenom and venom cause flaccid paralysis in cabbage looper (Table 1) and flesh fly larvae. In mouse bioassays, prevenom and venom are equally potent on a protein basis when administered through the intracerebroventricular route. However, venom is five times more lethal than prevenom on a volumetric basis (per microliter of venom injected). Prevenom administration initially causes hyperactivity in mice, whereas venom-injected animals immediately display akinesia and then convulsions. At later times, the symptoms of prevenom and venom are indistinguishable. If a sublethal dose of 10 μg of protein is injected through the s.c. route however, the symptoms of prevenom and venom are different. Administration of prevenom into the hind paw of mouse results in pain, as quantified by foot licking (Table 1). The symptoms include intense foot licking, foot shaking, and hyperactivity as long as 1 h after injection. When touched on the foot, prevenom-injected mice retract immediately, whereas venom-injected mice do not respond. Additionally, recovery from prevenom injection takes longer than that of venom. In contrast, when venom is administered, mice display reduced motor activity, become less mobile, and show less pain response (i.e., lick their feet threefold less compared with venom injected mice; see Table 1).
Prevenom Is More Efficacious but Less Potent than Venom.
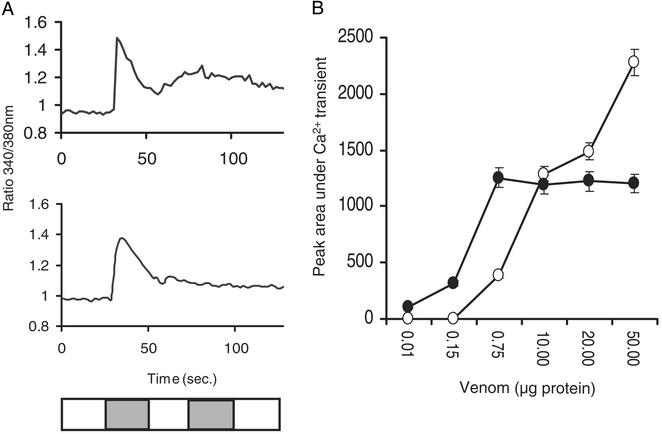
To gain a better understanding of the effects of prevenom and venom at the cellular level, the macroscopic Ca2+ transient of C2C12 skeletal myotubes are observed immediately after perfusion of prevenom or venom. The assay involved two sequential test solutions separated by a washout. After a 30-sec perfusion of wash medium (40 mM K+, control), prevenom or venom was introduced for 30 sec. During this test, K+ induces a very rapid Ca2+ transient that quickly decays to baseline. Prevenom- and venom-induced Ca2+ transients are distinctly different from that induced by K+ having slower onset and decays. Moreover, the prevenom is more efficacious because it induces Ca2+ transients that, on average, are twofold greater in magnitude than those induced by venom (Fig. 4A). This difference is, in large part, contributed by a more sustained rise in Ca2+ within the myotubes exposed to prevenom, which requires twice the time to decay to baseline compared with transients elicited by venom. In contrast, venom induces Ca2+ transients at substantially lower doses than prevenom, indicating the higher potency of venom. After a 30-sec washout, a second test solution containing 40 mM K+ failed to produce a substantial response in myotubes exposed to prevenom or venom, whereas control myotubes robustly responded. The responses to prevenom and venom are both dose-dependent (Fig. 4B).
Figure 4.
Cellular effects of prevenom and venom. (A) Prevenom and venom are applied on mouse skeletal cells and Ca2+ transient is quantified. The white blocks on the bottom bar represent the wash periods between the applications: the first shaded block represents the time frame of prevenom, venom, or high K+ application for the control; the second shaded block represents high K+ challenge. The responses are normalized to control high K+ response. Prevenom induces higher Ca2+ transients and the duration of the prevenom effect is two times longer than that of venom. Venom induces smaller Ca2+ transients but does so even at lower doses than prevenom. (B) Dose–response analysis of prevenom (black) and venom (white). The prevenom and venom signals are standardized through PCA analysis; the areas under each peak are calculated and plotted against the dose of prevenom or venom applied. Each data point is averaged from 20–35 cells.
Discussion
The implications of having a prevenom are severalfold. One implication is conservation of the metabolically more expensive venom. Having to inject a venom heavily loaded with protein in every instance regardless of the actual demand (i.e., level of threat) would require higher quantities of venom to be stored and also a faster rate of production. The rate of protein synthesis clearly can be limited by the availability of the resources. Thus, the scorpion may deplete venom more frequently, decreasing its chances for survival. Similarly, storage of highly disulfide-bridged and compact peptide toxins seems to be problematic. For example, in vitro expression of scorpion toxins almost invariably results in minute quantities of active peptide (14). Therefore, keeping a prevenom ready would undoubtedly be an important aspect of conserving by reducing the unnecessary use of venom.
Chemical analysis of prevenom shows that it contains about sixfold less protein and sixteenfold higher concentration of K+ salt than venom. Metabolically speaking, K+ salt is likely to be less expensive to the scorpion compared with peptide toxins. In cells, the intracellular K+ concentration is about 20- to 30-fold higher than that in the extracellular environment. Considering the volume of the venom gland, making available a concentrated, low-volume K+ solution should be very rapid. It is reasonable to expect that prevenom would be easier to replenish than venom which requires protein synthesis and folding. It seems that the scorpion exchanges a highly valuable asset with a less valuable one.
Another distinction between the prevenom and the venom is their mechanism of action. At the cellular level, prevenom is pharmacologically more efficacious than venom, because prevenom can depolarize myotubes at least twofold more efficiently than venom at equivalent high doses (Fig. 4). On the other hand, venom continues to depolarize myotubes at low doses, whereas prevenom has no effect, indicating the higher potency of venom. Accordingly, prevenom and venom have different patterns of depolarizing activity. For example, the repolarization after prevenom application takes about two times longer than that of venom (Fig. 4). At the organismal level, we show that the prevenom is at least 2.8-fold more “paralytic” to insects on a protein basis. Prevenom is more efficacious toward mammals in terms of inducing pain as well. However, venom is more lethal against both insects and mammals on a volume basis (Table 1). The high extracellular K+ salt concentration shifts the K+ equilibrium potential locally, and this alone may result in pain and minor contraction of the target organism. This equilibrium potential is very rapidly reversed by the action of a group of ion channels, including the rectifying K+ channels (24). Utilization of high K+ salt and peptide toxins that inhibit rectification simultaneously is expected to increase the extent and the duration of depolarization and thus synergize toxicity. This strategy is quite an unusual one, because it translates to two discrete types of venom from a single animal. To our knowledge, this is the first known example of a strategy where peptides and K+ salt are used together. Additionally, it is also in agreement with the functional role of prevenom that possibly requires high efficacy but not necessarily high potency. The synergism of K+ salt and peptide toxins is a concept that resembles the strategy used by cone snails that employ “toxin cabals,” synergistic pairs of toxins to obtain rapid paralysis (1, 2). In the case of scorpions, however, a portion of the peptides are substituted by inexpensive K+ salt.
A further interesting connection of prevenom to scorpion biology is the “sexual sting” phenomenon in scorpion mating. Male scorpions sting female counterparts multiple times during the courtship and mating process (25, 26). The exact purpose of the sexual sting is not yet known. The sexual sting could be a stimulating impulse or a predation suppressant. However, it seems to be an important aspect of the mating process in scorpions. It is likely that prevenom is secreted during the sexual sting. Given the mechanism of action of prevenom, the sexual sting may be used by the male scorpion to modify muscular contractions during the courtship and mating process without exposing his mate to venom components that in some species are highly toxic to scorpions.
The desired effect of venom from the perspective of the scorpion is likely to be severalfold. Under natural conditions for routine encounters, it may be advantageous for a scorpion to deter a predator and/or make an impression by causing intense pain. Alternatively, immobilizing a small arthropod just enough to subdue and initiate feeding on it is also advantageous. For example, prevenom may distract a mammal because of its pain causing and “hyperactivating” abilities, giving the scorpion an opportunity to escape. On the other hand, in cases where its life is in danger, the scorpion may need to defend itself with the utmost urgency, thus justifying the presence of a more deadly mixture, the venom. In general, it seems that prevenom may be sufficient for many routine encounters (19). Furthermore, the fact that the numbers of human fatalities caused by scorpion envenomations are far below the number of reported and unreported stings in regions where medically important scorpions are found, indicates that most stings may be limited with prevenom or an intermediate level of venom secretion (i.e., the transition from prevenom to venom) rather than a full sting.
The higher efficacy of prevenom shows that scorpions use the rapid and more efficient type of venom initially for a quick defensive maneuver and switch to the more potent venom if threat persists. This strategy shows that scorpions posses prevenom not only for conservation of venom but also for achieving increased biological activity. One explanation for the source of prevenom could be that a high K+ salt solution is the predescendant of venom in animals. For example, bee venom is also a transparent solution that does not contain the 6- to 7-kDa Na+ channel peptide toxins that are found in scorpion venom. However, it is highly efficient in inducing intense pain. An interesting similarity between bee venom and scorpion prevenom is that bees target K+ channels mainly with apamin, and scorpion prevenom achieves a similar response again by modulating K+ channels. Because of their role in regulating excitability, K+ channels are a strategic target exploited by numerous venomous animals including bees, scorpions, and snakes. It is hypothesized that short chain neurotoxins and long chain neurotoxins in venom are related to each other, and gene duplication may be responsible for the evolution of longer peptide toxins (27). We observed that small peptides of 900–1,200 Da in size are more prevalent than other components; the second most abundant group of peptides is the K+ channel blockers (3- to 4-kDa peptides), followed by the 6- to 7-kDa peptides classically targeting the Na+ channels that are the least abundant group in the prevenom. It is noteworthy to mention that this pattern is in agreement with the general evolutionary formula of “from simple to more complex” (28).
Further research may look at other venomous species to see if prevenom is common in other venomous animals as well. It will also be important to investigate the composition of prevenom in more detail and identify each component. Pairs of synergistic toxins from prevenom may then be found and coexpressed in recombinant baculoviruses for enhanced pest control. Regardless of the future applications, the presence and use of a prevenom in scorpions is a fascinating example of adaptation and survival ability on the part of scorpions, which also gives us a clue about why scorpions remain among the most successful of animal groups.
Supplementary Material
Acknowledgments
We thank the National Institute on Environmental Health Sciences (NIEHS) Superfund Basic Research Program (P42 ES04699) Analytical Core for analytical support. This work was funded in part by U.S. Department of Agriculture Competitive Research Grants Program 2001-35302-09919 and the NIEHS P30 ES05707.
References
- 1.Baldomero M O, Lourdes J C. Toxicon. 2001;39:7–14. [Google Scholar]
- 2.Baldomero M O. Mol Biol Cell. 1997;8:2101–2109. doi: 10.1091/mbc.8.11.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polis G A. In: The Biology of Scorpions. Polis G A, editor. Stanford, CA: Stanford Univ. Press; 1990. pp. 247–293. [Google Scholar]
- 4.Brownell P, Polis G. In: Scorpion Biology and Research. Brownell P, Polis G, editors. New York: Oxford Univ. Press; 2001. pp. 3–13. [Google Scholar]
- 5.Keegan H L. In: Scorpions of Medical Importance. Keegan H L, editor. Jackson: Univ. Press of Mississippi; 1980. p. 43. [Google Scholar]
- 6.Balozet L. In: Venomous Animals and Their Venoms. Vol. 3, Venomous Invertebrates. Bücherl W, Buckley E E, editors. New York: Academic; 1971. pp. 349–371. [Google Scholar]
- 7.Bücherl W. In: Venomous Animals and Their Venoms. Vol. 3, Venomous Invertebrates. Bücherl W, Buckley E E, editors. New York: Academic; 1971. pp. 317–347. [Google Scholar]
- 8.Zlotkin E. In: Arthropod Venoms. Bettini S, editor. New York: Springer; 1978. pp. 317–369. [Google Scholar]
- 9.Possani L D, Becerrill B, Delepierre M, Tytgat J. Eur J Biochem. 1999;264:287–300. doi: 10.1046/j.1432-1327.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 10.Becerril B, Marangoni S, Possani L D. Toxicon. 1997;35:821–835. doi: 10.1016/s0041-0101(96)00198-5. [DOI] [PubMed] [Google Scholar]
- 11.Tytgat J, Chandy K G, Garcia M L, Gutman G A, Martin-Eauclaire M–F, van der Walt J J, Possani L D. Trends Pharmacol Sci. 1999;20:444–447. doi: 10.1016/s0165-6147(99)01398-x. [DOI] [PubMed] [Google Scholar]
- 12.Fajloun Z, Kharrat R, Chen L, Lecomte C, Di Luccio E, Bichet D, El Ayeb M, Rochata H, Allen P D, Pessah I N, et al. FEBS Lett. 2000;469:179–185. doi: 10.1016/s0014-5793(00)01239-4. [DOI] [PubMed] [Google Scholar]
- 13.Corzo G, Escoubas P, Villegas E, Barnham K J, He W, Norton R S, Nakajima T. Biochem J. 2001;359:35–45. doi: 10.1042/0264-6021:3590035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turkov M, Rashi S, Noam Z, Gordon D, Khalifa R B, Stankiewicz M, Pelhate M, Gurevitz M. Protein Expression Purif. 1997;10:123–131. doi: 10.1006/prep.1997.0724. [DOI] [PubMed] [Google Scholar]
- 15.Rein J O. J Arachnology. 1993;21:60–63. [Google Scholar]
- 16.Prendini L. Zool Scripta. 2001;30:13–35. [Google Scholar]
- 17.Bergman N J. Toxicon. 1997;35:759–771. doi: 10.1016/s0041-0101(96)00041-4. [DOI] [PubMed] [Google Scholar]
- 18.Huys I, Dyason K, Waelkens E, Verdonck F, van Zyl J, du Plessis J, Müller G J, van der Walt J, Clynen E, Schoofs L, Tytgat J. Eur J Biochem. 2002;(269):1854–1865. doi: 10.1046/j.1432-1033.2002.02833.x. [DOI] [PubMed] [Google Scholar]
- 19.Yahel-Niv A, Zlotkin E. Toxicon. 1979;17:435–446. doi: 10.1016/0041-0101(79)90277-0. [DOI] [PubMed] [Google Scholar]
- 20.Inceoglu B, Lango J, Wu J, Hawkins P, Southern J, Hammock B D. Eur J Biochem. 2001;268:5407–5413. doi: 10.1046/j.0014-2956.2001.02479.x. [DOI] [PubMed] [Google Scholar]
- 21.Russell R M, Robertson J L, Savin N E. Bull Entomol Soc Am. 1971;23:209–213. [Google Scholar]
- 22.Fessenden J D, Wang Y, Moore R A, Chen S R W, Allen P D, Pessah I N. Biophys J. 2000;79:2509–2525. doi: 10.1016/S0006-3495(00)76492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson J E. A User's Guide to Principal Components. New York: Wiley Interscience; 1991. [Google Scholar]
- 24.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 25.Tallarovic S K, Melville J M, Brownell P H. J Insect Behav. 2000;13:827–838. [Google Scholar]
- 26.Polis G A, Sissom W D. In: The Biology of Scorpions. Polis G A, editor. Stanford, CA: Stanford Univ. Press; 1990. pp. 161–223. [Google Scholar]
- 27.Ceard B, Martin-Eauclaire M F, Bougis P E. FEBS Lett. 2001;494:246–248. doi: 10.1016/s0014-5793(01)02336-5. [DOI] [PubMed] [Google Scholar]
- 28.Trifonov E N, Kirzhner A, Kirzhner V M, Berezovsky I N. J Mol Evol. 2001;53:394–401. doi: 10.1007/s002390010229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.