Abstract
Germline mutations in SDHD predispose to the development of head and neck paragangliomas, and phaeochromocytomas. The risk of developing a tumor depends on the sex of the parent who transmits the mutation: paragangliomas only arise upon paternal transmission. In this study, both the risk of paraganglioma and phaeochromocytoma formation, and the risk of developing associated symptoms were investigated in 243 family members with the SDHD.D92Y founder mutation. By using the Kaplan–Meier method, age-specific penetrance was calculated separately for paraganglioma formation as defined by magnetic resonance imaging (MRI) and for paraganglioma-related signs and symptoms. Evaluating clinical signs and symptoms alone, the penetrance reached a maximum of 57% by the age of 47 years. When MRI detection of occult paragangliomas was included, penetrance was estimated to be 54% by the age of 40 years, 68% by the age of 60 years and 87% by the age of 70 years. Multiple tumors were found in 65% and phaeochromocytomas were diagnosed in 8% of paraganglioma patients. Malignant paraganglioma was diagnosed in one patient (3%). Although the majority of carriers of a paternally inherited SDHD mutation will eventually develop head and neck paragangliomas, we find a lower penetrance than previous estimates from studies based on predominantly index cases. The family-based study described here emphasizes the importance of the identification and inclusion of clinically unaffected mutation carriers in all estimates of penetrance. This finding will allow a more accurate genetic counseling and warrants a ‘wait and scan' policy for asymptomatic paragangliomas, combined with biochemical screening for catecholamine excess in SDHD-linked patients.
Keywords: paraganglioma, phaeochromocytoma, inheritance, SDHD, penetrance
Introduction
Paragangliomas of the head and neck are rare, usually benign tumors that arise in the paraganglion tissue associated with the parasympathetic nervous system.1 The carotid body in the carotid bifurcation is most frequently affected, followed by the jugulo-tympanic bodies at the jugular bulb and tympanic nerve, and the vagal bodies at the ganglions of the vagal nerve. Symptoms are usually mild and tumor progression is characteristically slow, and therefore diagnosis of the disease is often not made before adulthood.2 An estimated 10–50% of head and neck paragangliomas are hereditary.3 The natural course of the disease in hereditary cases does not seem to be different from sporadic paragangliomas, but patients with inherited disease are more likely to develop multiple paragangliomas.4 Hereditary head and neck paragangliomas can be caused by germline mutations in several genes encoding subunits of the mitochondrial succinate dehydrogenase (SDH) complex: the SDHB, SDHC and SDHD gene.5, 6, 7 SDHB (1p36.1-p35), encodes a catalytic subunit, whereas SDHC (1q21) and SDHD (11q23) encode membrane-anchoring subunits of SDH involved in electron transport. Furthermore, a yet unidentified gene (PGL2) on 11q13.1 causes paraganglioma in at least one family.8 Mutations in SDHB, SDHC and SDHD are also associated with the development of (extra-) adrenal paragangliomas or phaeochromocytomas.9, 10, 11, 12 As paragangliomas can cause incapacitating symptoms, accurate disease risk estimates are of paramount importance in clinical decision making and genetic counseling of paraganglioma patients. The chance of developing disease is dependent on the gene that is affected: in SDHB- and SDHC-linked families, inheritance is autosomal dominant, whereas in SDHD- and PGL2-linked families, the inheritance pattern shows a parent-of-origin effect.6, 12 As a rule, individuals are at risk only when they inherit the mutant SDHD allele from the father (regardless of his clinical status) and not when the mutation is maternally inherited.13, 14 Thus, proper genetic counseling of SDHD-linked paraganglioma families requires knowledge about the risk of developing head and neck paraganglioma upon paternal transmission of a SDHD mutation (penetrance), the risk of developing clinical symptoms, the age at onset of the disease, the risk of developing multiple tumors and the risk of developing phaeochromocytoma. To date, two reports have discussed the risk of developing paraganglioma or phaeochromocytoma upon inheritance of a SDHD mutation.15, 16 Both studies found that no tumors developed after maternal transmission of the SDHD mutation, and that penetrance of disease was 100% at the age of 70 years upon paternal transmission. However, both studies have evaluated a heterogeneous population of SDHD mutation carriers with different SDHD mutations, relatively large numbers of index cases (16 different SDHD mutations in 19 index patients and 15 different SDHD mutations in 24 index cases, respectively) and small numbers of asymptomatic family members.15, 16 This study design is prone to overestimation of penetrance if the index cases are selected from families with multiple affected individuals and if insufficient asymptomatic family members are included. Moreover, different mutations may confer different risks.17 In this study, we have therefore evaluated age-specific risk of developing a paraganglioma and/or phaeochromocytoma in an extended family consisting of 243 family members, in which the D92Y germline mutation in the SDHD gene segregates. As a significant number of paragangliomas remain asymptomatic even at advanced ages, the age-specific risk of developing paraganglioma-related symptoms is as important in counseling paraganglioma patients as the age-specific risk of developing a paraganglioma. For that reason, we have evaluated the penetrance of symptomatic disease and the penetrance of tumor development separately.
Materials and methods
Clinical status
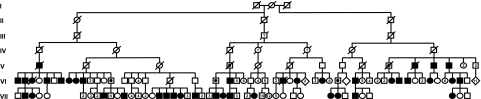
The disease status of 243 relatives belonging to a seven-generation family with head and neck paragangliomas (family FGT189) was established between 1990 and 2008. The pedigree of this family has been published before and was updated for this study (Figure 1).18, 19, 20 For the evaluation of clinical characteristics, data from the family members of generations V, VI and VII were used, because patients in older generations were not available for adequate clinical analysis. Family members from generation V, VI and VII underwent magnetic resonance imaging (MRI) of the head and neck if they showed signs or symptoms of paragangliomas. All non-symptomatic carriers of a paternal mutation, who were identified during genetic counseling, were offered clinical evaluation and MRI screening as well. In addition, the data acquired in a previous research protocol were used, in which 83 members of this family were examined with MRI, regardless of their disease status and sex of the carrier parent.18 To detect occult phaeochromocytomas, head and neck paraganglioma patients were biochemically screened for catecholamine excess. If screening was positive, MRI of the abdomen and 123I-MIBG scintigraphy was performed.
Figure 1.
Pedigree of family FGT189. Roman numerals correspond to the subsequent generations of the FGT189 family. Squares depict males, circles depict females and diamonds depict multiple siblings of both sexes. Open symbols represent unaffected family members, dotted symbols represent unaffected obligate carriers of a paternally inherited SDHD mutation and solid symbols represent affected family members. A question mark within a symbol stands for a possibly affected family member, as inferred from carrier status of offspring. A number within a symbol indicates the number of siblings.
Age at onset
As initial symptoms can be very mild and growth of paragangliomas is usually slow, there may be a substantial delay before a patient comes under medical attention. It can therefore be difficult to establish the exact age at onset of paraganglioma formation. We have defined ‘age at onset' as the age at onset of complaints and/or symptoms, that is, the age at which the patient retro spectively first experienced complaints of a head and neck paraganglioma or phaeochromocytoma, as opposed to the age at diagnosis, that is, the age at which the patient came under medical attention and the diagnosis of paraganglioma and/or phaeochromocytoma was established.
Genetic status
Paraganglioma patients in family FGT189 were shown to harbor the D92Y missense mutation (g.7882 T>C; Asp92Tyr) in the SDHD gene.21 This Dutch founder mutation was detected by direct sequencing of PCR products obtained from peripheral blood lymphocyte DNA, as described previously.7
Penetrance
For penetrance calculations, only the data from generations VI and VII were used, because insufficient data were available to identify asymptomatic mutation carriers in older generations (Figure 1). In this way, the risk of bias in penetrance calculations is minimized. Given the typical inheritance pattern of SDHD-linked paragangliomas, children of female mutation carriers were considered not to be at risk.13, 22 For children of affected fathers, the risk of inheriting the mutation was estimated the to be 50%. Penetrance was calculated by comparing the actual number of patients with and without symptoms with the expected number of family members at risk of inheriting the mutation.23 Next, we combined genetic and clinical data to calculate age-related penetrance in this family. Penetrance was expressed as a Kaplan–Meier curve, representing the probability of a SDHD mutation carrier to have developed either paraganglioma-related signs or symptoms, or a detectable paraganglioma at a given age. Calculations were performed for symptomatic and asymptomatic paragangliomas confirmed by radiology and for symptomatic paragangliomas alone.
Results
Clinical status
Figure 1 shows the pedigree of family FGT189. In generations V, VI and VII, paragangliomas were diagnosed in 40 family members (25 men, 15 women). Seven of these individuals (18%) had no signs or symptoms, and their diagnosis was made only after MRI screening. Multiple tumors were present in 26/40 patients (65%), to a maximum of five per patient. The most frequently encountered location was the carotid body (29 patients, 39 tumors) followed by jugulo-tympanic tumors (20 patients, 23 tumors) and the vagal body (13 patients, 16 tumors). Furthermore, 3/40 patients (8%) with head and neck paragangliomas also had an adrenal phaeochromocytoma as diagnosed by 123I-MIBG scintigraphy and MRI. In 1/40 paraganglioma patients (3%), metastatic paraganglioma tissue was found in the lung and spinal column, and this tumor was thus classified as malignant paraganglioma.
Age at onset of symptoms
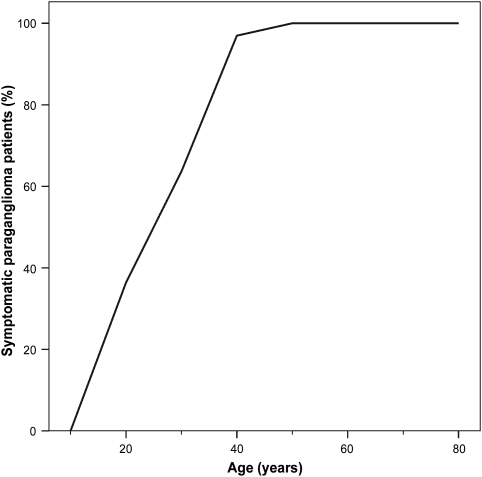
In generations V, VI and VII, 33 paraganglioma patients experienced symptoms. The age at onset of symptoms ranged from 14 to 47 years (mean 26.5 years; 95% CI, 23.5–29.6 years), with a mean delay of 2.6 years (95% CI, 1.4–3.7 years) until diagnosis (Figure 2).
Figure 2.
Age at onset of symptoms in SDHD-linked paraganglioma patients. Cumulative chart of the age at which the first symptoms of a head and neck paraganglioma or phaeochromocytoma became evident in the symptomatic family members in generation V, VI and VII (n=33).
Genetic status
In generation VI and VII, a total of 211 family members were alive at ascertainment and in Mendelian line of inheriting the D92Y founder mutation in the SDHD gene. Of these 211, 22 asymptomatic family members declined the invitation to be tested for the mutation. A total of 63 of the remaining 189 family members tested positive, 52 of whom inherited the mutation from their father. One Of the 22 asymptomatic family members who could not be tested was identified as an obligate carrier of a paternally inherited mutation, because of affected offspring. In all, 53 paternal and 11 maternal mutation carriers were thus identified in generation VI and VII.
Penetrance
As expected, penetrance of the disease was parent-of-origin dependent. No paragangliomas were found by clinical investigation or MRI in the offspring of female SDHD mutation carriers, and they were therefore not included in the risk calculations. We identified 11 male mutation carriers in generation V and 17 in generation VI on the basis that they were either affected themselves or had an affected offspring. In total, we identified 138 children of male mutation carriers (83 in generation VI and 55 in generation VII), who were thus all at 50% risk of inheriting the SDHD mutation. Of the 138 family members, 36 (26%) had one or more radiologically proven head and neck paragangliomas and/or a phaeochromocytoma. A total of 30 of these 36 patients (83%) experienced symptoms at the time of diagnosis or developed symptoms in the follow-up period. Under the assumption that 50% of the children of paternal mutation carriers are at risk, this corresponds with an estimated overall penetrance of 36/69 (52%) and an estimated overall clinical penetrance of 30/69 (43%).23
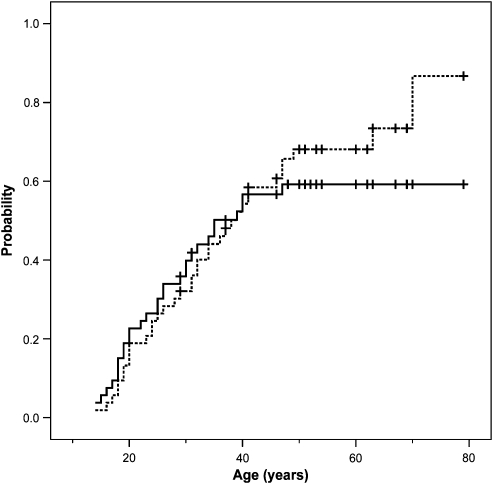
Using the genetic data, 53 of the 138 children (38%) at risk of a paternally transmitted mutation in generation VI and VII were shown to actually have inherited the mutation (Table 1). A total of 30 of these carriers at risk presented with paraganglioma or phaeochromocytoma-related symptoms, accounting for an overall clinical penetrance of 30/53 (57%). To correct for the age at onset, a Kaplan–Meier curve was made representing the chance to be symptom free as a function of time (Figure 3). As none of the carriers developed symptoms after the age of 47 years, the penetrance reached a maximum of 57% at this age. Of the 23 clinically non-penetrant carriers of the disease gene, 12 were examined with MRI. In six cases (50%), one or more paragangliomas were diagnosed, raising the overall penetrance to 36/53 (68%) (Table 1). If these cases were included in a Kaplan–Meier curve, the penetrance increased to 87% by 70 years of age (Figure 3).
Table 1. Penetrance in generations VI and VII.
| Carriers of a paternally inherited SDHD mutation (n) | Symptomatic paraganglioma patients (n) | MRI-diagnosed paraganglioma patients (n) | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | Total | Male | Female |
| 53 | 31 | 22 | 30 (57%) | 18 | 12 | 36 (68%) | 21 | 15 |
Figure 3.
Penetrance of SDHD-linked head and neck paragangliomas. Inverted Kaplan–Meier curve indicating the probability of developing a MRI-detectable paraganglioma (dotted line) or the probability of developing paraganglioma-related symptoms (solid line) at a certain age for carriers of a paternally inherited SDHD mutation. Vertical markers indicate censored patients.
Discussion
SDHD-linked paragangliomas and phaeochromocytomas present a unique tumor syndrome with a specific, parent-of-origin-dependent risk of inheriting disease. In this study, we did not observe the development of paragangliomas or phaeochromocytomas in 11 instances of maternal transmission of the SDHD mutation. This parent-of-origin-dependent inheritance seems to be the norm in SDHD-linked paraganglioma families, although one case of a tympanic paraganglioma after maternal transmission has been reported.13, 14 Recently, new insights in the mechanisms behind this peculiar inheritance pattern have emerged. We have shown previously that in SDHD-linked paragangliomas, not only the wild-type maternal SDHD allele on 11q23 but the entire maternal copy of chromosome 11 was consistently lost.22 A model explaining the parent-of-origin-dependent inheritance in SDHD-linked cases was proposed, involving a second, paternally imprinted, tumor-suppressor gene (TSG) located on 11p15.22 Within this model, paraganglioma formation occurs only when the wild-type maternal SDHD allele on 11q23 and the active copy of the imprinted TSG on 11p15 are simultaneously lost. This model of inheritance has been supported by the report of Pigny et al,24 who describe the only known case of maternal transmission of SDHD-linked paraganglioma to date. Although at first sight, this unique case seems to contradict the model, it was shown that the patient had also acquired an altered methylation profile and, therefore, probably an altered imprinted status of H19, a known paternally imprinted TSG on 11p15.24 This suggests that the parent-of-origin-dependent inheritance of SDHD-linked disease is caused by the paternal imprinting of H19, a TSG on the imprinted 11p15 region that seems to be essential for paraganglioma formation. It furthermore suggests that maternal transmission of SDHD-linked disease is possible only if the ‘second hit' targets the wild-type paternal SDHD allele on 11q23 as well as the active status of the maternal H19 allele on 11p15. The fact that these events involve different regions of different copies of chromosome 11 simultaneously is likely to be the reason why maternal transmission of disease is extremely rare.
This has considerable consequences on the genetic counseling of the affected families. Although children of female mutation carriers may not be completely preserved from the risk of developing paraganglioma, maternal transmission of disease remains extremely rare. Offspring of female carriers must, however, be aware that they can be mutation carriers and transmit the disease gene to their children. On the other hand, carriers of a paternally inherited SDHD mutation are at risk of developing a head and neck paraganglioma and/or phaeochromocytoma. In this study, the overall risk of developing a paraganglioma upon paternal transmission of the SDHD mutation is 68%, when evaluating the results of clinical evaluation and MRI. Age-related penetrance is 54% at the age of 40 years and 68% at the age of 60 years, reaching a maximum of 87% by the age of 70 years, that is, the large majority of patients with a paternally derived disease gene will eventually develop one or more paragangliomas and/or a phaeochromocytoma (Figure 3). These figures may represent an underestimation, because MRI scanning and screening for catecholamine excess was declined by 11 of the 23 asymptomatic paternal mutation carriers. Even in the unlikely event that all these would have had one or more occult paragangliomas or a phaeochromocytoma, overall penetrance is raised only marginally up to a maximum of 89%. Hence, this cannot fully explain why our estimates are slightly lower compared with those reported in the literature. Benn et al reported an estimated age-related penetrance for SDHD-linked disease of 73% at the age of 40 years and 100% at the age of 70 years, whereas Neumann et al reported a penetrance of 86% at the age of 50 years and 100% at the age of 70 years.15, 16 In the latter two studies, multiple families with multiple index patients and different SDHD mutations were investigated. In contrast, we have evaluated a single extended family with the D92Y Dutch founder mutation in the SDHD gene for the calculation of penetrance in SDHD-linked disease.15, 16 There is evidence that this family-based approach yields more accurate estimates, because less index cases and more asymptomatic mutation carriers are included.25 However, bias may arise if family members share unknown genetic or environmental factors that influence disease risk.25, 26 This could lead to overestimation of penetrance in SDHD-linked disease, especially when high-risk families are used for penetrance calculations.25, 27 In this respect, it is interesting to note that age-related penetrance estimates of SDHD-linked disease found in this study are lower compared with those reported by Benn et al and Neumann et al.15, 16 This may reflect an upward bias in the latter two studies because of the inclusion of large numbers of index cases and relatively low numbers of asymptomatic mutation carriers. Further positive bias may have arisen in these studies because all risk factors tend to be overrepresented in case patients.28
The risk of developing head and neck paraganglioma and/or phaeochromocytoma, or penetrance of the disease, is not the only feature that is important in counseling SDHD mutation carriers. The risk of developing associated symptoms is at least as relevant in counseling and clinical decision making. In this study, evaluation of the age-related occurrence of clinical symptoms upon paternal transmission of a SDHD mutation reveals that a significant number of individuals at risk did not develop clinical symptoms despite the fact that some of them have reached advanced ages. In actual fact, no patients developed first symptoms after the age of 47 years, and clinical penetrance reaches a maximum of 57% at this age (Figure 3). Clinical penetrance might even have been overestimated in our study, because 21 asymptomatic family members at risk of inheriting the SDHD mutation through the father were not tested for the SDHD mutation and their carrier status could not be inferred from their offspring. Assuming that 50% of these 21 untested family members would have inherited the mutation, the clinical penetrance decreases to 50%. In the unlikely event that all 21 untested family members would have inherited the SDHD mutation, clinical penetrance is at least 41%.
Astrom et al observed that patients with multiple SDHD-linked tumors or a concurrent phaeochromocytoma at the time of diagnosis had lived at higher mean altitudes as compared with those with single tumors.29 They postulated that the low altitudes found in the western part of the Netherlands cause a milder disease phenotype, manifesting as reduced penetrance and a better fitness of SDHD mutations. This would explain the relatively high incidence of hereditary paragangliomas and the remarkable clustering of founder mutations in the SDHD gene in the Netherlands.29 However, despite the fact that most family members of family FGT189 live in the western part of the Netherlands, a region situated at sea level, multiple tumors were ascertained in 65% of its patients, as compared with 30–74% in other studies.15, 16, 29 In addition, the risk of developing phaeochromocytoma is 8% for paraganglioma patients in this family, at the lower end of the spectrum of published risk estimates (7–53%), but comparable with that of patients living at higher altitudes (10%).10, 15, 16, 29 Remarkably, Astrom et al did not observe an effect of altitude on age at onset, although age-dependent carotid body hyperplasia at high altitudes has been observed by others.29, 30 However, they did find a correlation between age at onset and mutation type.29 Patients harboring missense mutations in the SDHD gene seemed to develop symptoms later in life than those harboring nonsense or splicing mutations (mean age at onset of 34.3 years versus 25.8 years, respectively).29 In the present family, patients harboring the D92Y missense mutation had a mean age at onset of 26.5 years (95% CI, 23.5–29.6 years), which was in good agreement with other studies that have evaluated paraganglioma patients with different SDHD mutations (25.8–30.6 years).15, 16, 29 Malignancy or metastatic disease, a rare finding in SDHD-linked disease with an estimated prevalence of 0–10%, has not been associated with residential altitude, nor with mutation type.15, 16, 29, 31 In the present family too, only one patient (3%) was diagnosed with a paraganglioma metastasis. All in all, the disease phenotype of the SDHD.D92Y mutation in this extended family residing at sea level does not seem to represent a milder or otherwise different phenotype than that of patients living at high altitudes or those carrying other mutation types, and hence altitude is unlikely to explain the observed lower penetrance. Probably, more relevant is the fact that the SDHD.D92Y missense mutation has a detrimental effect on the functionality of the SDH-complex.32 Moreover, as most patients develop first symptoms after reaching reproductive age, and symptoms are usually mild and slowly progressive even at high altitudes, it is doubtful whether negative selection plays a decisive role in the geographical distribution of SDHD mutations. Rather, the high incidence of founder mutations in the Netherlands is explained by specific historic and demographic factors, such as migrational patterns, endogamy and rapid population growth, factors that have contributed to the existence of a striking number of Dutch founder mutations in other disease genes.33
In summary, we have provided risk estimates for a well-defined SDHD-linked population and have shown that penetrance of disease differs considerably depending on whether or not MRI-screening results are included. Whereas the large majority of paternally inherited SDHD mutation carriers may eventually develop one or more paragangliomas (87%), symptoms do not occur in substantial proportion of these carriers at risk (43%), probably because of the characteristic indolent growth pattern of paragangliomas.2 Patients who do develop complaints associated with paraganglioma or phaeochromocytomas generally do so before the age of 50 years; the risk of developing symptoms later in life seems small. This knowledge might reassure especially older non-symptomatic carriers and warrants a ‘wait and scan' policy for patients with asymptomatic head and neck paragangliomas. Because of the elevated risk of developing a phaeochromocytoma in SDHD-linked disease, surveillance should include screening for the detection of asymptomatic phaeochromocytomas.2, 10
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thank Marciano B Ferrier and Ekaterina S Jordanova for their help with the statistical analysis and the figures.
References
- Parry DM, Li FP, Strong LC, et al. Carotid body tumors in humans: genetics and epidemiology. J Natl Cancer Inst. 1982;68:573–578. [PubMed] [Google Scholar]
- Jansen JC, van den Berg R, Kuiper A, van der Mey AG, Zwinderman AH, Cornelisse CJ. Estimation of growth rate in patients with head and neck paragangliomas influences the treatment proposal. Cancer. 2000;88:2811–2816. [PubMed] [Google Scholar]
- McCaffrey TV, Meyer FB, Michels VV, Piepgras DG, Marion MS. Familial paragangliomas of the head and neck. Arch Otolaryngol Head Neck Surg. 1994;120:1211–1216. doi: 10.1001/archotol.1994.01880350023005. [DOI] [PubMed] [Google Scholar]
- Struycken PM, Cremers CWRJ, Mariman ECM, Joosten FBM, Bleker RJTM. Glomus tumours and genomic imprinting: influence of inheritance along the paternal or maternal line. Clin Otolaryngol. 1997;22:71–76. doi: 10.1046/j.1365-2273.1997.00884.x. [DOI] [PubMed] [Google Scholar]
- Astuti D, Latif F, Dallol A, et al. Mutations in the mitochondrial complex II subunit SDHB cause susceptibility to familial phaeochromocytoma and paraganglioma. J Med Genet. 2001;38:S22. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann S, Muller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26:268–270. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- Mariman ECM, van Beersum SEC, Cremers CWRJ, van Baars FM, Ropers HH. Analysis of a second family with hereditary non-chromaffin paragangliomas locates the underlying gene at the proximal region of chromosome-11Q. Hum Genet. 1993;91:357–361. doi: 10.1007/BF00217356. [DOI] [PubMed] [Google Scholar]
- Peczkowska M, Cascon A, Prejbisz A, et al. Extra-adrenal and adrenal pheochromocytomas associated with a germline SDHC mutation. Nat Clin Pract Endocrinol Metab. 2008;4:111–115. doi: 10.1038/ncpendmet0726. [DOI] [PubMed] [Google Scholar]
- van Houtum WH, Corssmit EP, Douwes Dekker PB, et al. Increased prevalence of catecholamine excess and phaeochromocytomas in a well-defined Dutch population with SDHD-linked head and neck paragangliomas. Eur J Endocrinol. 2005;152:87–94. doi: 10.1530/eje.1.01833. [DOI] [PubMed] [Google Scholar]
- van Schothorst EM, Jansen JC, Grooters E, et al. Founder effect at PGL1 in hereditary head and neck paraganglioma families from the Netherlands. Am J Hum Genet. 1998;63:468–473. doi: 10.1086/301951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti D, Latif F, Dallol A, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mey AGL, Maaswinkelmooy PD, Cornelisse CJ, Schmidt PH, van de Kamp JJP. Genomic imprinting in hereditary glomus tumors – evidence for new genetic theory. Lancet. 1989;2:1291–1294. doi: 10.1016/s0140-6736(89)91908-9. [DOI] [PubMed] [Google Scholar]
- Mariman ECM, van Beersum SEC, Cremers CWRJ, Struycken PM, Ropers HH. Fine mapping of a putatively imprinted gene for familial nonchromaffin paragangliomas to chromosome 11Q13.1 – evidence for genetic-heterogeneity. Hum Genet. 1995;95:56–62. doi: 10.1007/BF00225075. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Pawlu C, Peczkowska M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292:943–951. doi: 10.1001/jama.292.8.943. [DOI] [PubMed] [Google Scholar]
- Benn DE, Gimenez-Roqueplo AP, Reilly JR, et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab. 2006;91:827–836. doi: 10.1210/jc.2005-1862. [DOI] [PubMed] [Google Scholar]
- Gayther SA, Mangion J, Russell P, et al. Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet. 1997;15:103–105. doi: 10.1038/ng0197-103. [DOI] [PubMed] [Google Scholar]
- van Gils AP, van der Mey AG, Hoogma RP, et al. MRI screening of kindred at risk of developing paragangliomas: support for genomic imprinting in hereditary glomus tumours. Br J Cancer. 1992;65:903–907. doi: 10.1038/bjc.1992.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterwijk JC, Jansen JC, van Schothorst EM, et al. First experiences with genetic counselling based on predictive DNA diagnosis in hereditary glomus tumours (paragangliomas) J Med Genet. 1996;33:379–383. doi: 10.1136/jmg.33.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schothorst EM, Jansen JC, Bardoel AF, et al. Confinement of PGL, an imprinted gene causing hereditary paragangliomas, to a 2-cM interval on 11q22–q23 and exclusion of DRD2 and NCAM as candidate genes. Eur J Hum Genet. 1996;4:267–273. doi: 10.1159/000472213. [DOI] [PubMed] [Google Scholar]
- Taschner PEM, Jansen JC, Baysal BE, et al. Nearly all hereditary paragangliomas in the Netherlands are caused by two founder mutations in the SDHD gene. Genes Chromosomes Cancer. 2001;31:274–281. doi: 10.1002/gcc.1144. [DOI] [PubMed] [Google Scholar]
- Hensen EF, Jordanova ES, van Minderhout IJHM, et al. Somatic loss of maternal chromosome 11 causes parent-of-origin-dependent inheritance in SDHD-linked paraganglioma and phaeochromocytoma families. Oncogene. 2004;23:4076–4083. doi: 10.1038/sj.onc.1207591. [DOI] [PubMed] [Google Scholar]
- van Baars FM, Cremers CWRJ, van den Broek P, Veldman JE. Familiar non-chromaffinic paragangliomas (glomus tumors) – clinical and genetic-aspects (abridged) Acta Otolaryngol. 1981;91:589–593. doi: 10.3109/00016488109138545. [DOI] [PubMed] [Google Scholar]
- Pigny P, Vincent A, Cardot BC, et al. Paraganglioma after maternal transmission of a succinate dehydrogenase gene mutation. J Clin Endocrinol Metab. 2008;93:1609–1615. doi: 10.1210/jc.2007-1989. [DOI] [PubMed] [Google Scholar]
- Gong G, Whittemore AS. Optimal designs for estimating penetrance of rare mutations of a disease-susceptibility gene. Genet Epidemiol. 2003;24:173–180. doi: 10.1002/gepi.10219. [DOI] [PubMed] [Google Scholar]
- Baysal BE. Genomic imprinting and environment in hereditary paraganglioma. Am J Med Genet C. 2004;129C:85–90. doi: 10.1002/ajmg.c.30018. [DOI] [PubMed] [Google Scholar]
- Choi YH, Kopciuk KA, Briollais L. Estimating disease risk associated with mutated genes in family-based designs. Hum Hered. 2008;66:238–251. doi: 10.1159/000143406. [DOI] [PubMed] [Google Scholar]
- Begg CB. On the use of familial aggregation in population-based case probands for calculating penetrance. J Natl Cancer Inst. 2002;94:1221–1226. doi: 10.1093/jnci/94.16.1221. [DOI] [PubMed] [Google Scholar]
- Astrom K, Cohen JE, Willett-Brozick JE, Aston CE, Baysal BE. Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Hum Genet. 2003;113:228–237. doi: 10.1007/s00439-003-0969-6. [DOI] [PubMed] [Google Scholar]
- Arias-Stella J, Valcarcel J. Chief cell hyperplasia in the human carotid body at high altitudes. Hum Pathol. 1976;7:361–373. doi: 10.1016/s0046-8177(76)80052-4. [DOI] [PubMed] [Google Scholar]
- Havekes B, Corssmit EP, Jansen JC, van der Mey AG, Vriends AH, Romijn JA. Malignant paragangliomas associated with mutations in the succinate dehydrogenase D gene. J Clin Endocrinol Metab. 2007;92:1245–1248. doi: 10.1210/jc.2006-1993. [DOI] [PubMed] [Google Scholar]
- Dekker PBD, Hogendoorn PCW, Kuipers-Dijkshoorn N, et al. SDHD mutations in head and neck paragangliomas result in destabilization of complex II in the mitochondrial respiratory chain with loss of enzymatic activity and abnormal mitochondrial morphology. J Pathol. 2003;201:480–486. doi: 10.1002/path.1461. [DOI] [PubMed] [Google Scholar]
- Zeegers MPA, van Poppel F, Vlietinck R, Spruijt L, Ostrer H. Founder mutation among the Dutch. Eur J Hum Genet. 2004;12:591–600. doi: 10.1038/sj.ejhg.5201151. [DOI] [PubMed] [Google Scholar]