Abstract
Oral estrogen administration attenuates the metabolic action of growth hormone (GH) in humans. To investigate the mechanism involved, we studied the effects of estrogen on GH signaling through Janus kinase (JAK)2 and the signal transducers and activators of transcription (STATs) in HEK293 cells stably expressing the GH receptor (293GHR), HuH7 (hepatoma) and T-47D (breast cancer) cells. 293GHR cells were transiently transfected with an estrogen receptor-α expression plasmid and luciferase reporters with binding elements for STAT3 and STAT5 or the β-casein promoter. GH stimulated the reporter activities by four- to sixfold. Cotreatment with 17β-estradiol (E2) resulted in a dose-dependent reduction in the response of all three reporters to GH to a maximum of 49–66% of control at 100 nM (P < 0.05). No reduction was seen when E2 was added 1–2 h after GH treatment. Similar inhibitory effects were observed in HuH7 and T-47D cells. E2 suppressed GH-induced JAK2 phosphorylation, an effect attenuated by actinomycin D, suggesting a requirement for gene expression. Next, we investigated the role of the suppressors of cytokine signaling (SOCS) in E2 inhibition. E2 increased the mRNA abundance of SOCS-2 but not SOCS-1 and SOCS-3 in HEK293 cells. The inhibitory effect of E2 was absent in cells lacking SOCS-2 but not in those lacking SOCS-1 and SOCS-3. In conclusion, estrogen inhibits GH signaling, an action mediated by SOCS-2. This paper provides evidence for regulatory interaction between a sex steroid and the GH/JAK/STAT pathway, in which SOCS-2 plays a central mechanistic role.
Growth hormone (GH) plays a major role in regulating somatic growth and substrate metabolism (1). It exerts the action via specific GH receptors (GHRs) in target tissues (2). GHR is a transmembrane protein, which, together with the receptors for prolactin and IL6, are members of the cytokine receptor family (3). Upon ligand binding, GHRs dimerize and induce activation and phosphorylation of Janus kinase (JAK)2 (4), which then phosphorylates GHRs and the signal transducers and activators of transcription (STATs), including STAT1, -3, and -5 (5). The STAT proteins dimerize and translocate to the nucleus, where they bind to specific DNA motifs within the promoter regions of GH-responsive genes to initiate transcription (6).
GH activation of the JAK/STAT pathway is negatively regulated by phosphotyrosine phosphatases and the suppressors of cytokine signaling (SOCS). Phosphatases, such as SHP1 and -2, inactivate JAK2 in a GH-dependent manner (7, 8). SOCS consists of a family of inhibitors, which suppress JAK/STAT signaling by a wide variety of cytokines, hormones, and growth factors (9). GH induces the expression of SOCS-1, -2, and -3, which feed back to inhibit its transcriptional action (10, 11).
There is strong evidence that estrogen negatively regulates GH action. Oral estrogen administration to women reduces serum levels of insulin-like growth factor I, despite elevating GH levels (12, 13), and suppresses GH stimulation of lipid oxidation (14). Moreover, women are less responsive than men to GH treatment (15). The mechanism of estrogen inhibition of GH action is unknown.
Estrogen action is mediated by specific nuclear estrogen receptors (ERs), which are ligand-activated transcription factors belonging to the steroid hormone receptor family (16). There is evidence of crosstalk between signaling pathways of steroid hormone and cytokine receptors. Ligand-bound glucocorticoid receptor (GR) directly associates with STAT5 and enhances prolactin-activated JAK/STAT signaling (17, 18).
In this study, we investigated the effects of estrogen on the transcriptional action of GH through the JAK/STAT pathway, and examined the roles of phosphotyrosine phosphatases and SOCS in estrogen regulation of GH action.
Materials and Methods
Reagents and Plasmids.
Recombinant human GH was produced in-house (19). Human prolactin and IL6 were obtained from National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda) and R & D Systems, respectively. ICI182780 was obtained from ICI. All cell culture reagents, calcium phosphate precipitation transfection kit, and TRIzol reagent were obtained from GIBCO/BRL. Omniscript reverse transcription kit and non-liposomal (Effectene) transfection reagent were obtained from Qiagen (Clifton Hill, Victoria, Australia). Lysis reagent and luciferase assay system were purchased from Promega (Madison, WI); Galacto Light was obtained from Tropix (Bedford, MA). Rabbit polyclonal antibodies against JAK2 (HR-758), STAT3 (KR-15), STAT5 (C-17), ERα (H-184), and GR (P-20) were obtained from Santa Cruz Biotechnology. The anti-phosphotyrosine monoclonal antibody (4G10) was obtained from Upstate Biotechnology (Lake Placid, NY). ImmunoPure Protein A/G agarose gel was obtained from Pierce. Complete protease inhibitor mixture (with inhibitors for serine, cysteine, metalloproteases, and calpains) and LightCycler-Fast Start Reaction Mix SYBR Green I were obtained from Roche Molecular Biochemicals. PolyScreen poly(vinylidene) difluoride membrane and Renaissance chemiluminescence reagent were obtained from NEN.
The human GHR expression plasmid (pcDNAI/Amp-GHRfl) was generated as reported (20). Expression plasmids for human ERα (pCMV-ERgly-neo), human STAT3, murine STAT5a (pCDNA3-mSTAT5a), and β-galactosidase (β-Gal; IEP-βgal-CMV) were provided by Craig Jordan (Robert H. Lurie Cancer Center, Chicago), James Darnell (The Rockefeller University, New York), Bernard Callus (Garvan Institute of Medical Research, Sydney) and Gerald Clesham (University of Cambridge, Cambridge, U.K.), respectively. Luciferase reporter constructs with three copies of m67, a high-affinity mutated form of c-sis-inducible element for STAT3 binding (5′-CTGCAGTCGACATTTCCCGTAAATCGTCGACTGCA-3′; pUC18-SIE m67/TK), or with six copies of a synthetic lactogenic hormone response element for STAT5 binding (5′-CTGCAGTGTGGACTTCTTGGAATTAAGGGACTTTTGCTGCAG-3′; pUC18-LHRE/TK) fused to a minimal thymidine kinase promoter (21) were provided by Paul Kelly (Faculte de Medecine Necker, Paris). Luciferase reporter constructs containing an NdeI-XhoI fragment of the rat β-casein promoter (nucleotides −344 to −1) in a pLucDSS plasmid (pZZ1; ref. 22) or a consensus estrogen response element (ERE; 5′-AGGTCACTGTGACCT-3′) in a minimal TK GL3 plasmid (pERE/TK/GL3; ref. 23) were provided by Bernd Groner (Institute of Experimental Cancer Research, Freiburg, Germany) and Malcolm Parker (Imperial Cancer Research Fund, London), respectively. PCR primers for human SOCS-1 (forward, 5′-AGACCCCTTCTCACCTCTTG-3′; reverse, 5′-CTGCACAGCAGAAAAATAAAGC-3′; ref. 24), SOCS-2 (forward, 5′-GGATGGTACTGGGGAAGTATGACTG-3′; reverse, 5′-AGTCGATCAGATGAACCACACTGTC-3′); and SOCS-3 (forward, 5′-TCCCCCCAGAAGAGCCTATTAC-3′; reverse, 5′-TCCGACAGAGATGCTGAAGAGTG-3′) were obtained from Sigma Genosys (Sydney). Double-stranded short interfering RNA (siRNA) fragment (5′-AAGACCCAGTCTGGGACCAAGAA-3′) for nucleotides 359–381 of the human SOCS-3 gene for RNA interference assay was obtained from Xeragon (Huntsville, AL).
Cell Cultures.
HEK293 cells stably expressing human GHR (293GHR; ref. 25) were routinely grown at 37°C in 5% CO2/95% air in DMEM/F-12 supplemented with 10% (vol/vol) FCS/25 mM Hepes/2 mM l-Gln/50 units per ml of penicillin/50 μg/ml streptomycin. HuH7, T-47D, and primary embryonic fibroblasts from SOCS-1- or SOCS-2-deficient (SOCS-1−/− and SOCS-2−/−, respectively) and wild-type (C57BL/6) mice (provided by Warren Alexander and Christopher Greenhalgh, Walter and Eliza Hall Institute of Medical Research, Melbourne; ref. 26) were cultured in Eagle's minimal essential medium, RPMI medium 1640, and DMEM, respectively. All cultures were changed to phenol red-free medium supplemented with 10% charcoal-stripped FCS for 3 days before use. All experiments were performed in phenol red-free medium under serum-free conditions.
Transcription Assays.
293GHR cells in 12-well multidishes were transiently transfected for 24 h with expression plasmids for ERα (0.1 μg) and β-Gal (0.01 μg), luciferase reporters with the STAT3- or STAT5-binding elements or with a rat β-casein promoter (0.1 μg) by using the calcium phosphate precipitation method. Expression plasmids for STAT3 (0.2 μg) and STAT5a (0.05 μg) were cotransfected in the STAT3 and β-casein reporter assays, respectively. The cells were then treated in triplicate with 500 ng/ml GH and varying concentrations of 17β-estradiol (E2) at 37°C for 6 or 18 h. Because dexamethasone (Dex) has been shown to enhance the STAT5 response to GH (27), 250 nM Dex was added in the STAT5 reporter assay. The cells were solubilized in lysis reagent [25 mM Tris, pH 7.8, with 2 mM trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, 2 mM DTT, 10% (vol/vol) glycerol, and 1% Triton X-100]. Luciferase and β-Gal activities were measured by the luciferase assay system and Galacto Light, respectively. Luciferase activity was normalized for β-Gal activity and reported as fold induction compared with the untreated control.
The effects of E2 on STAT5 reporter activation by GH were also examined in human hepatoma (HuH7) and breast cancer cells (T-47D), and in fibroblasts from wild-type, SOCS-1−/−, and SOCS-2−/− mice. Coexpression with GHR and ERα was performed in HuH7 cells and fibroblasts but not in T-47D cells, which express both receptors endogenously (28, 29). Transfection was carried out by using the Effectene reagent. The subsequent procedures of treatment and enzyme assays were the same as for 293GHR cells.
Western Analysis.
The effects of E2 on GH-induced phosphorylation of JAK2, STAT3, and STAT5 in 293GHR cells and on JAK2 phosphorylation in HuH7 cells and fibroblasts were examined by Western analysis. Cells expressing ERα (and GHR in HuH7 and fibroblasts) were pretreated with 100 nM E2 at 37°C for 2.5 h and then with 500 ng/ml GH for 2 min (JAK2) or 1 h (STAT3 and STAT5). The cells were washed with 1 mM sodium orthovanadate (vanadate) in PBS and solubilized in lysis buffer (50 mM Tris, pH 7.2/0.14 M NaCl/10 mM NaF/1 mM vanadate/0.4% Triton X-100) with Complete protease inhibitor mixture. Lysates were incubated with 2 μg of antibodies against JAK2, STAT3, or STAT5 at 4°C for 18 h and precipitated with ImmunoPure Protein A/G agarose gel. The samples were resolved by SDS/PAGE on 7.5% gel and blotted onto poly(vinylidene) difluoride membrane. The membrane was treated sequentially with blocking buffer (20 mM Tris⋅HCl, pH 7.4/150 mM NaCl/1% BSA/0.1% Tween-20), anti-phosphotyrosine antibody (4G10, 5 μg/5 ml), and sheep anti-mouse Ig-horseradish peroxidase. The bands were visualized by chemiluminescence and quantified by densitometry.
Protein abundance of JAK2, STAT3, and STAT5 was determined after stripping the membrane with 62.5 mM Tris⋅Cl, pH 6.8, 100 mM 2-mercaptoethanol, and 2% SDS at 50°C for 30 min. The membrane was treated with blocking buffer containing 5% skim milk powder in place of BSA and probed with 5 μg/5 ml of antibodies against JAK2, STAT3, or STAT5. The signal was developed with donkey anti-rabbit Ig-horseradish peroxidase by chemiluminescence and quantified by densitometry. Levels of phosphorylated JAK2 and STAT were corrected for protein abundance, and reported as percentages of control treated with GH alone.
SOCS mRNA.
The mRNA abundance of SOCS-1, -2, and -3 in HEK293 cells treated with E2 or GH was quantified by reverse transcription accompanied by real-time PCR. HEK293 cells expressing ERα and GHR were treated in triplicate with 100 nM E2 or 500 ng/ml GH for 0.25, 0.5, 1, 2, and 4 h. At the indicated time, total RNA was extracted by using TRIzol reagent. Reverse transcription was performed by using the Omniscript reverse transcription kit as recommended by the manufacturer, in which 0.5 μg of RNA and oligo-dT primer were used. Controls with no reverse transcriptase were included.
Standards for real-time PCR were constructed for the SOCS by PCR using respective primer sets and plasmids containing the human SOCS genes as templates (30, 31). Real-time PCR assays were carried out in a LightCycler (Roche Molecular Biochemicals) with a 20-μl final volume containing 2 μl of sample cDNA or standards, 5 mM MgCl2, 0.5 μM primers, and 2 μl of LightCycler-Fast Start Reaction Mix SYBR Green I. The amplification program included an initial denaturation step at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 0.5 s, annealing at 55°C for 5 s and extension at 72°C for 10 s, with ramping rates at 20°C/s. The specificity of amplification was subjected to melting curve analysis by heating from 65°C to 95°C at the rate of 0.1°C/s. Amplification curves were plotted as fluorescence signal against cycle number, and the first turning point (crossing point) was obtained for each sample by using the Second Derivative Maximum Method (Roche Molecular Biochemicals). Values of crossing point for standards were used to construct a calibration curve, from which copy numbers for the samples were estimated. All samples were assayed at the same time for statistical comparison.
SOCS-3 RNA Interference.
To examine whether SOCS-3 was involved in the E2 regulation, its expression was selectively suppressed by using the RNA interference method (32). HEK293 cells were transiently transfected with SOCS-3 siRNA (8 nM), expression plasmids for ERα, GHR, and β-Gal, and the STAT5 reporter by using the Effectene reagent. Cells cotransfected with an expression plasmid for human SOCS-3 (50 ng) were included to assess the efficiency of RNA interference.
Statistical Analysis.
All experiments were repeated three times unless stated otherwise. Mean ± SE of results from multiple experiments of the same study are reported. Data were analyzed by Student's paired t test or ANOVA (STATVIEW V.4.5, Abacus Concepts, Berkeley, CA) where appropriate, and significance was set at P < 0.05.
Results
STAT5, STAT3, and β-Casein Reporters in 293GHR.
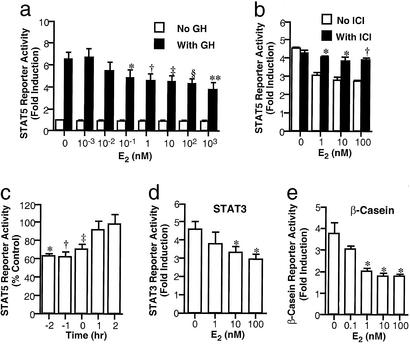
GH stimulated STAT5 reporter response by 6.8 ± 0.8 fold (n = 4; P < 0.01; Fig. 1a). E2 reduced the response in a dose-dependent manner, with significant inhibition at 0.1 nM (76 ± 6% of control; P < 0.05) and maximal reduction at 1,000 nM (59 ± 1%; P < 0.01). The inhibitory effect was not detected without ER expression (data not shown) and was attenuated by pretreatment with 1 μM ICI182780, an anti-estrogen (n = 4; P < 0.05; Fig. 1b).
Figure 1.
Effects of E2 on GH transcriptional activity in 293GHR cells. (a) Cells were transiently transfected with an ERα expression plasmid and STAT5 reporter and treated with 500 ng/ml GH and 250 nM Dex and E2 at indicated concentrations for 6 h. *, P < 0.05; †, P < 0.01. (b) Effect of anti-estrogen. After pretreatment for 30 min with 1 μM ICI182780, the cells were incubated with 500 ng/ml GH and 250 nM Dex and E2 for 6 h; vs. control with E2 at corresponding concentrations, *, P < 0.05. (c) Time course of E2 effect. The cells were treated with a 1-h pulse of 500 ng/ml GH, washed, and incubated in fresh medium for a further 5 h before luciferase activity measurement. E2 at 100 nM was added at indicated time before (minus) or after (plus) the GH pulse. For −2, −1, and 0 h, E2 was replaced in fresh medium during the 5-h incubation without GH. Control cultures without E2 were set up for each time point. Luciferase activity of E2-treated samples is presented as percentage of non-E2-treated control of the same time point; vs. controls at the same time point: *, P < 0.01; †, P < 0.05. (d) STAT3 reporter. Cells expressing exogenous STAT3 and ERα were transiently transfected with the STAT3 reporter and treated with 500 ng/ml GH and E2 at indicated concentrations for 6 h. *, P < 0.05. (e) β-Casein promoter reporter. Cells expressing exogenous STAT5a and ERα were transiently transfected with the β-casein promoter reporter and treated with 500 ng/ml GH and 250 nM Dex and E2 for 24 h. *, P < 0.05.
To study the time course of E2 inhibition, 293GHR cells were treated with 100 nM E2 before, during, and after a 1-h pulse of GH. Pretreatment with E2 for 1 and 2 h reduced the reporter response to 62 ± 6% and 63 ± 3% of control, respectively (n = 4; P < 0.01; Fig. 1c). E2 added together with GH had a lesser effect (71 ± 6%; P < 0.05), whereas no inhibition was detected with E2 addition after the GH pulse.
Fig. 1d shows the effect of E2 on GH transcriptional action mediated by STAT3. As with STAT5, E2 reduced the STAT3 reporter response to GH in a dose-dependent manner from 4.3 ± 0.4-fold to 2.8 ± 0.2-fold at 100 nM (n = 4; P < 0.05).
To determine whether E2 inhibition occurred on a natural promoter, we examined the effect on GH activation of a β-casein promoter reporter in 293GHR cells expressing exogenous STAT5a. GH induced the reporter activity by 3.8 ± 0.5-fold (P < 0.01; Fig. 1e). Cotreatment with E2 reduced the response to 49 ± 4% of control at 100 nM (P < 0.05). Similar results were observed in T-47D cells expressing exogenous STAT5a (data not shown).
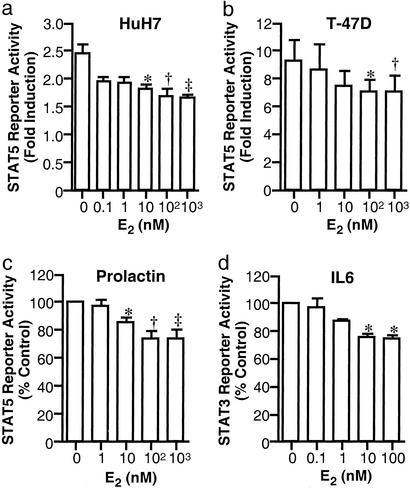
STAT5 Reporter in HuH7 and T-47D.
To examine whether E2 inhibited GH transcriptional action in other cell types, studies were performed in HuH7 (hepatoma) and T-47D (breast cancer) cells. In HuH7 cells coexpressing GHR and ERα, GH activated the STAT5 reporter by 2.3 ± 0.2-fold (n = 8; P < 0.01). The response was inhibited by E2, with a maximal reduction to 76 ± 7% of control at 1,000 nM (P < 0.01; data not shown). Similarly, E2 at 100 nM reduced the GH-induced response in T-47D cells from 9.3 ± 1.5-fold to 7.0 ± 1.2-fold (n = 4; P < 0.01; data not shown).
Prolactin and IL6 Signaling.
Next, we studied the effects of E2 on JAK/STAT signaling of other cytokines, namely prolactin and IL6. In T-47D cells which express endogenous prolactin receptors (33), prolactin stimulated STAT5 reporter activity by 2.8 ± 0.4-fold (n = 4; P < 0.05). The response was reduced by E2 to 74 ± 4% of control (P < 0.05; Fig. 2a).
Figure 2.
Effects of E2 on transcriptional activities of prolactin and IL6. (a) T-47D cells transfected with the STAT5 reporter were treated for 24 h with 500 ng/ml prolactin and 250 nM Dex and E2. *, P < 0.05. (b) 293GHR cells transfected with the STAT3 reporter and expression plasmids for ERα and STAT3 were treated for 24 h with 500 ng/ml IL6 and E2. *, P < 0.05.
The effect on IL6 signaling was studied in HEK293 cells with endogenous IL6 receptor and exogenous STAT3 (27). IL6 activated the STAT3 reporter by 1.8 ± 0.1-fold (P < 0.01), and 100 nM E2 reduced the response to 75 ± 2% of control (P < 0.05; Fig. 2b).
ERE Reporter.
Having demonstrated the inhibitory effect of E2 on GH signaling, we examined whether GH exerted a reciprocal effect on E2 action by using an ERE reporter. E2 increased the reporter activity in a dose-dependent manner to a maximum of 1.6 ± 0.1-fold and 10.9 ± 2.8-fold at 100 nM in 293GHR and T-47D cells, respectively (n = 4; P < 0.01). GH at 500 ng/ml did not affect the stimulation at any concentration of E2 (data not shown).
Dex-Enhanced STAT5 Reporter Activity.
As Dex was used in the STAT5 reporter assay to enhance response (17), we examined whether the effect of E2 depended on the presence of glucocorticoid. GH-induced STAT5 activity was significantly lower in the absence than the presence of 250 nM Dex (9.1 ± 0.6-fold and 19.6 ± 1.0-fold, respectively; P < 0.01). However, the reporter response to GH was inhibited to a similar extent by 1,000 nM E2 without or with Dex (55 ± 2% and 58 ± 1% of control, respectively; P < 0.01; data not shown).
STAT Phosphorylation.
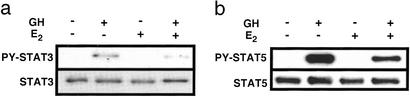
To elucidate the mechanism of E2 inhibition, its effects on GH-induced phosphorylation of STAT3 and STAT5 were studied by Western blotting (Fig. 3). GH stimulation of STAT phosphorylation was reduced by E2 to 62 ± 7% (P < 0.05) and 50 ± 4% of GH-treated control (P < 0.01), respectively. Neither GH nor E2 affected protein abundance of the STATs.
Figure 3.
Western blotting of tyrosine phosphorylation (PY) of STAT3 (a) and STAT5 (b) in 293GHR cells. The cells were treated for 2.5 h with 100 nM E2 and then for 1 h with 500 ng/ml GH (also with 250 nM Dex in the case of STAT5), as indicated. Cell lysates were immunoprecipitated with antibodies for STAT3 or STAT5 and Western blotted with an anti-phosphotyrosine antibody (Upper) and the anti-STAT antibodies (Lower).
Interaction of STAT5 with ER and GR.
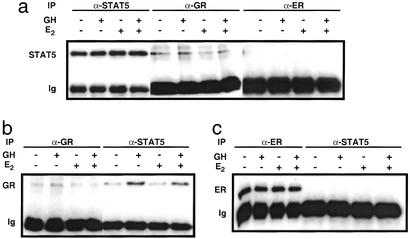
As GR directly associates with STAT5 to modulate its transcriptional activity (17), we investigated whether ERα bound to STAT5 by coimmunoprecipitation. As shown in Fig. 4a, STAT5 was coprecipitated with an anti-GR antibody, revealing association of STAT5 with GR. Similarly, GR was coprecipitated with an anti-STAT5 antibody, and the association was enhanced by GH treatment (Fig. 4b). In contrast, the STAT5 band was not detected in the precipitates of an antibody to ERα (Fig. 4a), nor was ERα coprecipitated with an anti-STAT5 antibody (Fig. 4c).
Figure 4.
Coimmunoprecipitation of STAT5 with GR and ERα in 293GHR cells. Western blots of STAT5 (a), GR (b), and ERα (c) in 293GHR cells treated for 1 h with 250 nM Dex, without or with 500 ng/ml GH and 100 nM E2, and precipitated (IP) with antibodies against STAT5, GR, or ERα, as indicated. The bottom bands (Ig) were the heavy chains of the antibodies used for immunoprecipitation.
JAK2 Phosphorylation.
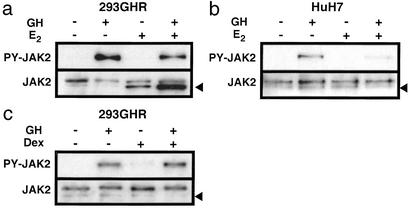
Having demonstrated that E2 exerted similar effects on the activation of STAT3 and STAT5, we examined whether the inhibition resulted from suppression of JAK2 phosphorylation. GH markedly induced JAK2 phosphorylation in 293GHR (Fig. 5a) and HuH7 cells (Fig. 5b), and E2 reduced this response to 50 ± 3% and 48 ± 3% of control, respectively (n = 6; P < 0.01). In contrast to E2, Dex did not affect JAK2 phosphorylation in 293GHR cells (Fig. 5c).
Figure 5.
Regulation of GH-induced phosphorylation of JAK2 by E2 in 293GHR (a) and HuH7 (b) cells, or by Dex in 293GHR cells (c). The cells were treated for 2.5 h with 100 nM E2 or 250 nM Dex and then for 2 min with 500 ng/ml GH as indicated, followed by immunoprecipitation for JAK2 and Western blotting for phosphorylated (PY) and total JAK2. Arrowheads indicate nonspecific bands.
Actinomycin D.
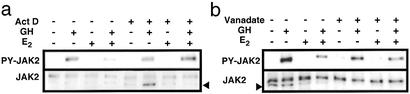
As ERα is a transcription factor, we studied whether de novo gene expression was required for the E2 inhibition. As shown previously, E2 decreased GH-induced JAK2 phosphorylation to 52 ± 7% of control in the absence of actinomycin D (Fig. 6a). Pretreatment for 1 h with 5 μg/ml actinomycin D completely abolished this effect (95 ± 5% of control), suggesting that E2 inhibition of GH signaling is indirectly mediated and requires the expression of another factor(s).
Figure 6.
Effects of actinomycin D and vanadate in 293GHR cells. Western blots of GH-induced tyrosine phosphorylation (PY) of JAK2 in 293GHR cells treated for 1 h with 5 μg/ml actinomycin D (a; Act D) or 1 mM vanadate (b), followed by treatment for 2.5 h with 100 nM E2 and for 2 min with 500 ng/ml GH, as indicated. Arrowheads indicate nonspecific bands.
Vanadate.
We examined the role of phosphotyrosine phosphatases in E2 inhibition by using vanadate, a general inhibitor of phosphatases (34, 35). Pretreatment for 1 h with 1 mM vanadate did not affect E2 inhibition of GH-induced JAK2 phosphorylation (48 ± 4% vs. 43 ± 6%, without and with vanadate, respectively; Fig. 6b). In the STAT5 reporter assay, vanadate did not affect the inhibitory effect of E2 at concentrations up to 100 nM (data not shown). Thus, phosphotyrosine phosphatases do not appear to be involved in the E2 inhibition.
SOCS.
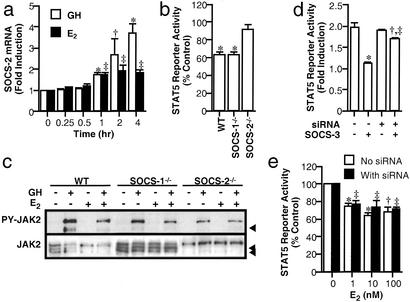
Next, we investigated the role of SOCS proteins by studying the effects of E2 on expression of SOCS-1, -2, and -3. In HEK293 cells expressing GHR and ERα, SOCS-2 mRNA abundance increased significantly to 1.7 ± 0.1-fold of control at 1 h of GH treatment (P < 0.01; Fig. 7a) and rose progressively to 3.7 ± 0.3-fold at 4 h (P < 0.01). E2 also induced a time-dependent increase in SOCS-2 mRNA abundance by 1.7 ± 0.1-fold at 1 h (P < 0.01), and no further increase was observed thereafter. The mRNA abundance of SOCS-1 and -3 was unaffected by GH or E2 treatment (data not shown).
Figure 7.
SOCS. (a) Effects of E2 and GH on SOCS-2 expression in HEK293 cells expressing ERα and GHR. The cells were treated with 100 nM E2 or 500 ng/ml GH for time as indicated. SOCS-2 mRNA abundance was quantified by reverse transcription and real-time PCR; vs. respective control at time 0: *, P < 0.01; †, P < 0.05. (b) Effects of E2 (1 nM) on STAT5 activation by GH (500 ng/ml) in wild-type (WT) and SOCS-deficient fibroblasts expressing ERα and GHR. Results are expressed as percentages of GH-treated control for the respective cell lines. *, P < 0.01. (c) Western blots of GH-induced JAK2 phosphorylation (PY) in the fibroblasts treated for 2.5 h with 1 nM E2 and then for 2 min with 500 ng/ml GH, as indicated. Arrowheads indicate nonspecific bands. (d) Effects of SOCS-3 siRNA transfection on GH activation of STAT5 reporter in HEK293 cells expressing SOCS-3. The cells were transiently transfected with a GHR expression plasmid, a STAT5 reporter, with or without SOCS-3 siRNA (8 nM) and a SOCS-3 expression plasmid (50 ng), as indicated, and then treated for 3 h with 500 ng/ml GH and 250 nM Dex. Data shown are the mean ± SE of triplicate measurements in a representative experiment, which was repeated twice; vs. respective control without SOCS-3: *, P < 0.01, †, P < 0.05; vs. sample without siRNA and with SOCS-3: ‡, P < 0.01. (e) Effects of SOCS-3 siRNA on E2 inhibition of GH-induced STAT5 activity in HEK293 cells. Cells expressing ERα and GHR were cotransfected with or without SOCS-3 siRNA (8 nM) and treated for 3 h with 500 ng/ml GH and 250 nM Dex and E2 at indicated concentrations. Data shown are the mean ± SE of triplicate measurements in a representative experiment, which was repeated twice; vs. respective control: *, P < 0.01; †, P < 0.05.
Then, we examined the effects of E2 on GH activation of the STAT5 reporter and JAK2 phosphorylation in wild-type, SOCS-1−/−, and SOCS-2−/− fibroblasts. GH activated the reporter activity by three- to sixfold in these cells. In wild-type and SOCS-1−/− fibroblasts, the response was reduced by E2 to 63 ± 3% and 63 ± 4% of control, respectively (P < 0.01; Fig. 7b), but unchanged in SOCS-2−/− cells. E2 also suppressed GH-induced JAK2 phosphorylation in wild-type and SOCS-1−/− fibroblasts to 47 ± 3% and 50 ± 4% of control, respectively (P < 0.01; Fig. 7c), but not in SOCS-2−/− cells (98 ± 5%).
The role of SOCS-3 in E2 inhibition was studied by using the RNA interference method, as cells lacking the SOCS-3 gene are unavailable. Efficiency of this method was examined by transiently transfecting HEK293 cells with the SOCS-3 siRNA and expression plasmids for ERα, GHR, and SOCS-3. SOCS-3 expression fully blocked GH activation of the STAT5 reporter (2.0 ± 0.1-fold vs. 1.1 ± 0.1-fold; P < 0.01; Fig. 7d). The siRNA cotransfection increased the response to 90 ± 1% of control (P < 0.01). The RNA interference effect was specific for SOCS-3, as no effect on SOCS-1 and SOCS-2 on GH signaling was observed (data not shown).
The effect of SOCS-3 siRNA on E2 inhibition of GH action was examined next. E2 reduced the reporter response to a similar extent in cells with or without siRNA transfection (69 ± 6% and 64 ± 1% of control, respectively; P < 0.05; Fig. 7e). Thus, the collective data suggest that SOCS-2, rather than SOCS-1 and -3, is involved in the E2 inhibition.
Discussion
This study was instigated by clinical observations in our laboratory that oral estrogen administration antagonizes the metabolic action of GH (14, 36). We demonstrated that estrogen inhibited the transcriptional action of GH via the JAK/STAT pathway. The effect was ER-mediated and involved suppression of both STAT3 and STAT5 signaling. Similar inhibitory effects were detected for prolactin and IL6. The inhibition of GH action was effective only with prior exposure to estrogen. A direct interaction of ERα with STAT5 was not evident. Estrogen suppressed GH-induced phosphorylation of JAK2 and STATs, an effect not affected by phosphotyrosine phosphatase inhibition but abolished by actinomycin D, indicating a dependency on gene transcription. Estrogen increased the mRNA abundance of SOCS-2 but not of SOCS-1 or -3. The inhibition of GH signaling was absent in cells lacking SOCS-2 but not the other SOCS. This evidence demonstrates that a steroid hormone regulates SOCS expression, which in turn modulates GH signaling.
There are multiple sites for estrogen inhibition of GH action. Estrogen may reduce GHR availability by down-regulating receptor expression, or it may suppress GH signaling. Most previous studies focus on GHR expression as the mechanism and show that estrogen increases GHR mRNA level in rodents (37). The effect on human GHR is not clear. In our study, no effect of estrogen on GH binding was observed in 293GHR cells (unpublished observations). This observation is predictable because GHR expression in these cells is controlled by an exogenous viral promoter, which is not estrogen-responsive. These findings also led us to investigate the effects of estrogen on GH signaling via the JAK/STAT pathway.
We first examined possible association of ERα with STAT5 based on evidence of crosstalk between glucocorticoid and prolactin signaling occurring through direct interaction of GR with STAT5 (38). Stoecklin et al. (17) have shown that glucocorticoid enhances JAK/STAT activation by prolactin, and that the mechanism involves GR associating with and acting as a coactivator of STAT5. We extended these findings by showing that glucocorticoid enhancement of GH-induced STAT5 activation was associated with GR interaction with endogenous STAT5. On the other hand, we found no evidence for ERα interaction with STAT5, which suggests that estrogen inhibition of GH signaling is unlikely to arise from sequestering of STAT5 by ERα. As STAT5 binds to GR at the activation function 1 domain (39), a region known to be highly heterogeneous among steroid hormone receptors, the present findings suggest that ERα does not contain such an interactive site for STAT5. However, our findings are in conflict with the recent reports that ERs associate with exogenously expressed STAT3 (40) and STAT5 (41). The reason for the discrepancy is unclear but could relate to differences in experimental conditions, as the association of ERs with STAT3 and STAT5 was detected under conditions of STAT over-expression. It is unlikely that technical factors accounted for our inability to demonstrate this interaction because association between endogenous STAT5 and GR was observed under similar experimental conditions.
The observation that estrogen inhibited GH activation of STAT3 and STAT5 led us to examine whether this effect was exerted upstream at the level of JAK2 activation. The suppression of GH-induced JAK2 phosphorylation by estrogen stands in contrast to the effect of glucocorticoid, which is exerted at the level of STAT5 activation, an observation also reported by von Laue et al. (27). The collective data reveal that estrogen and glucocorticoid exert opposite effects on GH signaling through different mechanisms.
Estrogen inhibition of GH signaling was uniformly observed in all cell types tested, except SOCS-2−/− fibroblasts. Because most of these studies were performed after transfection with ERα and GHR, it is possible that the inhibition might be a squelching effect of estrogen resulting from competition of limited factors for gene transcription. However, this possibility is unlikely because similar inhibitory effects were detected in T-47D cells, which express endogenous ERs and GHRs. These data suggest that the inhibition of GH signaling is a specific effect of estrogen.
The study with actinomycin D revealed that the inhibition of GH signaling was not a direct effect of ERα. This finding is consistent with the view that estrogen functions mainly through effects on gene transcription (42). Results of the time course study also suggest indirect inhibition by a factor expressed in response to estrogen. Pretreatment with estrogen was required for effective inhibition of GH signaling, probably reflecting the time for synthesis of the factor.
Phosphotyrosine phosphatases and SOCS were considered likely candidates for mediating the inhibitory action of estrogen. The studies with vanadate, however, did not support a role for phosphotyrosine phosphatases. We then investigated whether SOCS proteins mediated the estrogen inhibition. Estrogen stimulated the expression of SOCS-2 in HEK293 cells. The stimulation was acute and significant by 1 h, and remained elevated by 4 h. GH also stimulated SOCS-2 expression, confirming and extending similar observations in rodents (10, 43). Unlike SOCS-2, we found no evidence for regulation of SOCS-1 and SOCS-3 expression by estrogen and GH in HEK293 cells. This observation of GH contrasts with previous reports that GH acutely and transiently stimulates expression of these two SOCS proteins in rat hepatocytes (10, 43). It is unclear whether the differences arise from tissue or species-specific effect.
The studies with SOCS-deficient cells and RNA interference revealed SOCS-2 and not SOCS-1 or -3 to be the key mediator of estrogen inhibition of GH signaling. There is strong evidence from gene knockout studies that SOCS-2 is an important negative regulator of GH action. SOCS-2−/− mice display a phenotype of excess growth (44) similar to that of GH-transgenic mice (45). The absence of a giant phenotype in the SOCS-2/STAT5b double-knockout mice (26) suggests the importance of SOCS-2 regulation of GH action mediated by the JAK/STAT pathway and hence the potential significance of estrogen inhibition.
The induction of GH resistance by estrogen may explain, in part, the gender differences in GH levels and responsiveness to GH treatment (15). Estrogen inhibition of the JAK/STAT signaling by prolactin and IL6 may have wider physiologic implications because of the pivotal role of this signaling pathway in mediating signal transduction of cytokine receptors (46). We speculate that the inhibition by estrogen of prolactin-induced lactation, the sexual dimorphism in red cell mass, and the modulatory role of estrogen in immune function may be underpinned by a broader role in regulating cytokine signaling involving SOCS protein.
In conclusion, estrogen inhibits GH signaling via the JAK/STAT pathway by suppressing JAK2 phosphorylation, an effect exerted through stimulation of SOCS-2. This is a mechanism of sex steroid regulation of GH action, and may have significance beyond estrogen and GH action.
Acknowledgments
We thank Mabrouka Maamra for performing the IL6 assay. This study was supported in part by the National Health and Medical Research Council of Australia and Eli Lilly Australia Pty. Ltd. Dr. C. K. W. Watts was supported by the New South Wales Cancer Council and United States Army Medical Research and Materiel Command Grant DAMD17-00-1-253; Dr. K. Sjogren was supported by the Wennergren Foundation, Sweden; and Dr. G. M. Leong was supported by the Vincent Fairfax Family Foundation, Australia.
Abbreviations
- GH
growth hormone
- GHR
GH receptor
- JAK
Janus kinase
- STAT
signal transducer and activator of transcription
- SOCS
suppressor of cytokine signaling
- ER
estrogen receptor
- GR
glucocorticoid receptor
- β-Gal
β-galactosidase
- siRNA
short interfering RNA
- E2
17β-estradiol
- Dex
dexamethasone
References
- 1.Davidson M B. Endocr Rev. 1987;8:115–131. doi: 10.1210/edrv-8-2-115. [DOI] [PubMed] [Google Scholar]
- 2.Waters M J. In: The Handbook of Physiology. Kostyo J L, Goodman H M, editors. Vol. 5. Oxford: Oxford Univ. Press; 1999. pp. 397–444. [Google Scholar]
- 3.Bazan J F. Proc Natl Acad Sci USA. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argetsinger L S, Campbell G S, Yang X, Witthuhn B A, Silvennoinen O, Ihle J N, Carter-Su C. Cell. 1993;74:237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- 5.Finidori J. Vitam Horm (San Francisco) 2000;59:71–97. doi: 10.1016/s0083-6729(00)59004-9. [DOI] [PubMed] [Google Scholar]
- 6.Herrington J, Smit L S, Schwartz J, Carter-Su C. Oncogene. 2000;19:2585–2597. doi: 10.1038/sj.onc.1203526. [DOI] [PubMed] [Google Scholar]
- 7.Hackett R H, Wang Y-D, Sweitzer S, Feldman G, Wood W I, Larner A C. J Biol Chem. 1997;272:11128–11132. doi: 10.1074/jbc.272.17.11128. [DOI] [PubMed] [Google Scholar]
- 8.Stofega M R, Herrington J, Billestrup N, Carter-Su C. Mol Endocrinol. 2000;14:1338–1350. doi: 10.1210/mend.14.9.0513. [DOI] [PubMed] [Google Scholar]
- 9.Kile B T, Alexander W S. Cell Mol Life Sci. 2001;58:1627–1635. doi: 10.1007/PL00000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams T E, Hansen J A, Starr R, Nicola N A, Hilton D J, Billestrup N. J Biol Chem. 1998;273:1285–1287. doi: 10.1074/jbc.273.3.1285. [DOI] [PubMed] [Google Scholar]
- 11.Ram P, Waxman D J. J Biol Chem. 1999;274:35553–35561. doi: 10.1074/jbc.274.50.35553. [DOI] [PubMed] [Google Scholar]
- 12.Weissberger A J, Ho K K Y, Lazarus L. J Clin Endocrinol Metab. 1991;72:374–381. doi: 10.1210/jcem-72-2-374. [DOI] [PubMed] [Google Scholar]
- 13.Kam G Y W, Leung K-C, Baxter R C, Ho K K Y. J Clin Endocrinol Metab. 2000;85:1918–1922. doi: 10.1210/jcem.85.5.6527. [DOI] [PubMed] [Google Scholar]
- 14.Wolthers T, Hoffman D M, Nugent A G, Duncan M W, Umpleby M, Ho K K Y. Am J Physiol. 2001;281:E1191–E1196. doi: 10.1152/ajpendo.2001.281.6.E1191. [DOI] [PubMed] [Google Scholar]
- 15.Burman P, Johansson A G, Siegbahn A, Vessby B, Karlsson F A. J Clin Endocrinol Metab. 1997;82:550–555. doi: 10.1210/jcem.82.2.3776. [DOI] [PubMed] [Google Scholar]
- 16.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoecklin E, Wissler M, Gouilleux F, Groner B. Nature. 1996;383:726–728. doi: 10.1038/383726a0. [DOI] [PubMed] [Google Scholar]
- 18.Wyszomierski S L, Yeh J, Rosen J M. Mol Endocrinol. 1999;13:330–343. doi: 10.1210/mend.13.2.0232. [DOI] [PubMed] [Google Scholar]
- 19.Ho K Y, Weissberger A J, Stuart M C, Day R O, Lazarus L. Clin Endocrinol. 1989;30:335–345. doi: 10.1111/j.1365-2265.1989.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 20.Ross R J M, Esposito N, Shen X Y, Von Laue S, Chew S L, Dobson P R M, Postel-Vinay M-C, Finidori J. Mol Endocrinol. 1997;11:265–273. doi: 10.1210/mend.11.3.9901. [DOI] [PubMed] [Google Scholar]
- 21.Sotiropoulos A, Moutoussamy S, Renaudie F, Clauss M, Kayser C, Gouilleux F, Kelly P A, Finidori J. Mol Endocrinol. 1996;10:998–1009. doi: 10.1210/mend.10.8.8843416. [DOI] [PubMed] [Google Scholar]
- 22.Gouilleux F, Wakao H, Mundt M, Groner B. EMBO J. 1994;13:4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowley S M, Parker M G. J Steroid Biochem Mol Biol. 1999;69:165–175. doi: 10.1016/s0960-0760(99)00055-2. [DOI] [PubMed] [Google Scholar]
- 24.Blumenstein M, Bowen-Shauver J M, Keelan J A, Mitchell M D. J Clin Endocrinol Metab. 2002;87:1094–1097. doi: 10.1210/jcem.87.3.8463. [DOI] [PubMed] [Google Scholar]
- 25.Maamra M, Finidori J, Von Laue S, Simon S, Justice S, Webster J, Dowers S, Ross R. J Biol Chem. 1999;274:14791–14798. doi: 10.1074/jbc.274.21.14791. [DOI] [PubMed] [Google Scholar]
- 26.Greenhalgh C J, Bertolino P, Asa S L, Metcalf D, Corbin J E, Adams T E, Davey H W, Nicola N A, Hilton D J, Alexander W S. Mol Endocrinol. 2002;16:1394–1406. doi: 10.1210/mend.16.6.0845. [DOI] [PubMed] [Google Scholar]
- 27.von Laue S, Finidori J, Maamra M, Shen X-Y, Justice S, Dobson P R M, Ross R J M. J Endocrinol. 2000;165:301–311. doi: 10.1677/joe.0.1650301. [DOI] [PubMed] [Google Scholar]
- 28.Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjold M, Gustafsson J-A. J Clin Endocrinol Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 29.Ballesteros M, Leung K C, Ross R J M, Iismaa T P, Ho K K Y. J Clin Endocrinol Metab. 2000;85:2865–2871. doi: 10.1210/jcem.85.8.6711. [DOI] [PubMed] [Google Scholar]
- 30.Dey B R, Spence S L, Nissley P, Furlanetto R W. J Biol Chem. 1998;273:24095–24101. doi: 10.1074/jbc.273.37.24095. [DOI] [PubMed] [Google Scholar]
- 31.Dey B R, Furlanetto R W, Nissley P. Biochem Biophys Res Commun. 2000;278:38–43. doi: 10.1006/bbrc.2000.3762. [DOI] [PubMed] [Google Scholar]
- 32.Elbashir S M, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 33.Shiu R P C. Cancer Res. 1979;39:4381–4386. [PubMed] [Google Scholar]
- 34.Swarup G, Cohen S, Garbers D L. Biochem Biophys Res Commun. 1982;107:1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- 35.Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser M J, Ramachandran C. J Biol Chem. 1997;272:843–851. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- 36.O'Sullivan A J, Crampton L J, Freund J, Ho K K Y. J Clin Invest. 1998;102:1035–1040. doi: 10.1172/JCI2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartzbauer G, Menon R K. Mol Genet Metab. 1998;63:243–253. doi: 10.1006/mgme.1998.2685. [DOI] [PubMed] [Google Scholar]
- 38.Stoecklin E, Wissler M, Schaetzle D, Pfitzner E, Groner B. J Steroid Biochem Mol Biol. 1999;69:195–204. doi: 10.1016/s0960-0760(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 39.Stoecklin E, Wissler M, Moriggl R, Groner B. Mol Cell Biol. 1997;17:6708–6716. doi: 10.1128/mcb.17.11.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto T, Matsuda T, Junicho A, Kishi H, Saatcioglu F, Muraguchi A. FEBS Lett. 2000;486:143–148. doi: 10.1016/s0014-5793(00)02296-1. [DOI] [PubMed] [Google Scholar]
- 41.Faulds M H, Pettersson K, Gustafsson J-A, Haldosen L-A. Mol Endocrinol. 2001;15:1929–1940. doi: 10.1210/mend.15.11.0726. [DOI] [PubMed] [Google Scholar]
- 42.Tsai M J, O'Malley B W. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 43.Tollet-Egnell P, Flores-Morales A, Stavreus-Evers A, Sahlin L, Norstedt G. Endocrinology. 1999;140:3693–3704. doi: 10.1210/endo.140.8.6878. [DOI] [PubMed] [Google Scholar]
- 44.Metcalf D, Greenhalgh C J, Viney E, Wilson T A, Starr R, Nicola N A, Hilton D J, Alexander W S. Nature. 2000;405:1069–1073. doi: 10.1038/35016611. [DOI] [PubMed] [Google Scholar]
- 45.Palmiter R D, Norstedt G, Gelinas R E, Hammer R E, Brinster R L. Science. 1983;222:809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- 46.Leonard W J, O'Shea J J. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]