Abstract
Photoreceptor apoptosis and resultant visual deficits occur in humans and animals with inherited and disease-, injury-, and chemical-induced retinal degeneration. A clinically relevant mouse model of progressive rod photoreceptor-selective apoptosis was produced by low-level developmental lead exposure and studied in combination with transgenic mice overexpressing Bcl-xL only in the photoreceptors. A multiparametric analysis of rod apoptosis and mitochondrial structure-function was performed. Mitochondrial cristae topography and connectivity, matrix volume, and contact sites were examined by using 3D electron tomography. Lead-induced rod-selective apoptosis was accompanied by rod Ca2+ overload, rhodopsin loss, translocation of Bax from the cytosol to the mitochondria, decreased rod mitochondrial respiration and membrane potential, mitochondrial cytochrome c release, caspase-3 activation, and an increase in the number of mitochondrial contact sites. These effects occurred without mitochondrial matrix swelling, outer membrane rupture, caspase-8 activation, or Bid cleavage. Bcl-xL overexpression completely blocked all apoptotic events, except Ca2+ overload, and maintained normal rod mitochondrial function throughout adulthood. This study presents images of mitochondrial contact sites in an in vivo apoptosis model and shows that Bcl-xL overexpression blocks increased contact sites and apoptosis. These findings extend our in vitro retinal studies with Pb2+ and Ca2+ and suggest that developmental lead exposure produced rod-selective apoptosis without mitochondrial swelling by translocating cytosolic Bax to the mitochondria, which likely sensitized the Pb2+ and Ca2+ overloaded rod mitochondria to release cytochrome c. These results have relevance for therapies in a wide variety of progressive retinal and neuronal degenerations where Ca2+ overload, lead exposure, and/or mitochondrial dysfunction occur.
Apoptotic photoreceptor cell death and visual deficits occur in developing and adult humans and animals with inherited as well as disease-, injury-, and chemical-induced retinal degeneration (1–9). The kinetics of rod apoptosis and decay of rod electroretinogram responses in patients and animals with slowly progressing retinal degeneration follow an exponential decline (10). This finding suggests that a single stochastic biochemical event such as Ca2+ overload or the generation of reactive oxygen species initiates or amplifies this catastrophic event (7, 10–12). Results showing that rat retinas incubated in Ca2+ and/or Pb2+ undergo rod-selective apoptosis by opening the mitochondrial permeability transition pore and activating the cytochrome c (Cyt c)-caspase cascade support this prediction (7). The aims of this in vivo study were to develop and characterize a pathophysiologically relevant model of progressive rod-selective apoptosis, to determine the mitochondrial mechanisms underlying the rod apoptosis, and to determine whether Bcl-xL overexpression preserved rod viability as well as mitochondrial ultrastructure, substructure, and bioenergetic function.
Bcl-2 and Bcl-xL overexpression prevent apoptosis in excitable and nonexcitable tissues/cells triggered by Ca2+ overload, Bax and Bak, reactive oxygen species as well as during neuronal development (13–18). The anti- and pro-apoptotic Bcl-2 family proteins are located in or translocated to the outer and inner mitochondrial membranes (OMM and IMM) or contact sites (CSs) (17–22). The mitochondrial localization of these proteins and their role in regulating the release of Cyt c across the OMM suggest that the antiapoptotic Bcl-2 family members exert their function by binding and regulating mitochondrial proteins located at the CSs (15, 19–22).
Here we show that low-level developmental lead exposure produced rod-selective apoptosis that was mediated by Bax translocation, decreased the mitochondrial membrane potential (ΔΨm), and caused Cyt c release and caspase-3 activation. Three-dimensional (3D) electron microscopic (EM) tomography revealed a lead-induced increase in the number of mitochondrial CSs without mitochondrial matrix swelling or outer membrane rupture. No evidence of Bid activation was found. Bcl-xL overexpression completely blocked all apoptotic events, and maintained normal rod mitochondrial structure and function. These results suggest that Bcl-xL exerts its antiapoptotic effect by blocking Bax translocation to the mitochondria.
Materials and Methods
Production and Identification of Bcl-xL Transgenic Mice.
The interphotoreceptor retinoid binding protein (IRBP)-bcl-xL transgene was constructed as described (23), except that the IRBP promoter was cloned immediately upstream of the bcl-xL transgene. The ClaI/XhoI fragment containing IRBP-bcl-xL was excised and purified for microinjection. Injected embryos were implanted into pseudopregnant CB6F1 female mice. Six lines of transgenic mice overexpressing Bcl-xL in photoreceptors were made. For all studies presented herein, line 3 was used as the founder had the highest level of Bcl-xL expression. The transgene in offspring was identified by Southern blot analysis (data not shown). Subsequent offspring were screened for the transgene by PCR. Whole mouse eyes were fixed overnight. Paraffin sections were cut, and Bcl-xL was detected by immunohistochemistry as described (23). Immunoblotting analysis confirmed the presence of the 28-kDa band of overexpressed Bcl-xL protein (data not shown).
Developmental Lead Exposure Model.
Bcl-xL transgenic female mice were mated with C57BL/6 control males. Pregnant mice were divided into control and lead-exposed groups. On the day of birth (postnatal day 0, PN0) through weaning (PN21), dams in the lead group received a 0.15% lead acetate drinking solution. Weaned pups received tap water and Purina chow ad libitum. The dams' food and fluid intake were not altered by lead exposure. All pups exhibited normal development. Animals were genotyped and divided into four groups: wild-type (control), Bcl-xL transgenic (Bcl-xL), wild-type/Lead (Lead), and Bcl-xL transgenic/Lead (Bcl-xL/Lead). Elemental lead ([Pb]) was measured (mean ± SEM; five to seven mice per age) as described (24). At PN21, blood and retinal [Pb] in controls were 2 ± 1 μg/dl and 0.02 ± 0.02 ppm, respectively, and in lead-exposed mice were 26 ± 5 μg/dl and 0.23 ± 0.04 ppm, respectively. At PN90, blood and retinal [Pb] in controls were 4 ± 2 μg/dl and 0.05 ± 0.02 ppm, respectively, and in lead-exposed mice were 6 ± 3 μg/dl and 0.10 ± 0.03 ppm, respectively. Rod outer–inner segment (OS–IS) [Ca] was measured in three to five dark-adapted mice at PN25 as described (24, 25).
To minimize the possibility that our results occurred during the assays because of increased Ca2+ and/or Pb2+ (7) or mitochondrial fragility (26), retinas were rinsed in an EGTA buffer and assays were run with and without 10 μM cyclosporin A, which blocks all Ca2+- and Pb2+-induced rod apoptosis (7). No differences were found, so the data were combined.
Histology, EM, and Rhodopsin Assay.
Counts of rod and cone cells (nuclei) were made in four retinal quadrants in PN7–180 mice (three to seven mice per age): central retina and far periphery of the superior and inferior temporal quadrants as described (2, 27). For EM, retinas from PN21 mice were immersion fixed and stained, and ultra-thin sections were examined in a JEOL 100-C or 1200EX as described (2, 5). Rhodopsin content (nmol) per eye was determined in PN90 mice (five to seven mice per group) as described (2, 27).
DNA Fragmentation.
To detect cleavage of genomic DNA into high molecular weight fragments, single cell retinal suspensions were made from each pair of PN21 mouse retinas and field inversion gel electrophoresis (FIGE; three to five experiments) was used as described (7). DNA fragments were visualized by UV fluorescence.
Determination of ΔΨm.
PN21 mouse retinas were stained with JC-1 (5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethylbenzimidazolycarbo-cyanine), whole mounted and examined with a fluorescence microscope (three to five experiments) as described (7). Pairs of digitized and processed images of the same rods from intact retinas showing red (585 nm) and green (520 nm) fluorescence were captured.
Rod Photoreceptor Oxygen Consumption (QOPR).
QOPR was recorded from isolated pairs of PN25 and PN90 mouse retinas. QOPR was determined polarographically and recorded continuously in the dark or during presentation of a rod-saturating light adapting stimulus as described (25, 28).
EM Tomography: Single- and Double-Tilt Series.
Three-dimensional tomographic reconstructions of rod mitochondria from immersion fixed PN21 mouse retinas were imaged in situ as described (29, 30). Three-dimensional reconstructions were obtained from semithick samples in a tilt-series every 2° from ±60° on a JEOL 4000EX operated at 400 kV. Double-tilt tomography provided more isotropic resolution (31). Ten different measurements of rod mitochondrial substructures were obtained: cristae widths, cristae junction diameters, OMM-IMM widths, CS widths, CS diameters, CS surface area, CS density, cristae/mitochondrial volume, cristae/mitochondrial surface area, and cristae segments per unit volume. Overall, measurements were from 61 different rod mitochondria with three or four mice per group.
Western Blot Analysis.
To detect Cyt c release, Bax translocation, and Bid cleavage, retinal cytosolic and mitochondrial fractions from PN21 control and lead-exposed mice were prepared (n = 3 per treatment) and the immunoblots were run as described (7). Blots were probed with an anti-Cyt c mouse monoclonal antibody (7), an anti-Bax monoclonal mouse antibody (mBax 5B7: gift from Yi-Te Hsu, Medical University of South Carolina, Charleston) and an anti-Bid/tBid polyclonal goat anti-human/mouse antibody (1 μg/ml; R & D Systems) followed by HRP-conjugated sheep anti-mouse (1:7,500; Amersham Pharmacia) and anti-goat IgG (1:2,000; Santa Cruz Biotechnology), respectively. The blots were developed with enhanced chemiluminescence reagents as described (7).
Malate Dehydrogenase (MDH) and Caspase Assays.
MDH-specific activity of lubrol-treated supernatant and mitochondria was measured essentially as described (32). Caspase-3 and -8 activity were measured by using N-acetyl-Asp-Glu-Val-Asp (DEVD)-p- nitroanilide and N-acetyl-Ile-Glu-Thr-Asp (IETD)-p-nitroanilide as substrates, respectively, as described (7). For each, three to five experiments on PN21 retinas per group were run.
Statistical Analysis.
All data analyses were performed by using an ANOVA and Fisher's protected least significant difference (PLSD) post hoc comparisons (statview, Abacus Concepts, Berkeley, CA). Differences from controls were regarded as significant if P < 0.05.
Results
Bcl-xL Transgenic Mice and Retinal Expression.
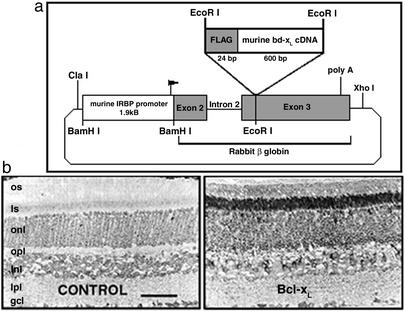
To date, rod-specific (rhodopsin promoter) overexpression of the Bcl-2 transgene in different mouse models of rapid and total rod degeneration has produced only a transient and partial rescue, or no rescue (4, 33). Moreover, in wild-type mice overexpressing Bcl-2 in photoreceptors there was a 20–50% loss of rods and rhodopsin (4, 34). To overcome these problems, a transgenic mouse with Bcl-xL overexpression under control of the murine IRBP promoter (Fig. 1) was used because the initial and maximal levels of IRBP expression, a glycolipoprotein unique to retina and pineal, occur earlier during postnatal development than opsin (35): the period when most rod apoptosis occurs in inherited and chemical-induced retinal degenerations (4, 6, 10, 12). In addition, a kinetically and clinically relevant mouse model of rod-selective apoptosis produced by developmental lead exposure was established (Fig. 2).
Figure 1.
Production and identification of Bcl-xL transgenic mice. (a) Map of the mouse IRBP-bcl-xL transgene construct, constructed as described in Materials and Methods. (b) Bcl-xL immunocytochemistry in wild-type (CONTROL) and Bcl-xL transgenic (Bcl-xL) mouse retinas. Control adult mouse neural retina have compact organization of rod and cone OS and IS, outer nuclear layer (onl; 10–11 nuclei), outer plexiform layer (opl), inner nuclear layer (inl; 4–5 nuclei), inner plexiform layer (ipl), and ganglion cell layer (gcl; 1–2 nuclei). In controls, low-level staining is observed in all nuclear layers and ISs. In Bcl-xL mice, the overexpressed protein is localized exclusively to photoreceptors such that intense staining is observed in the ISs and perinuclear (onl) region. (Scale bar = 50 μm.)
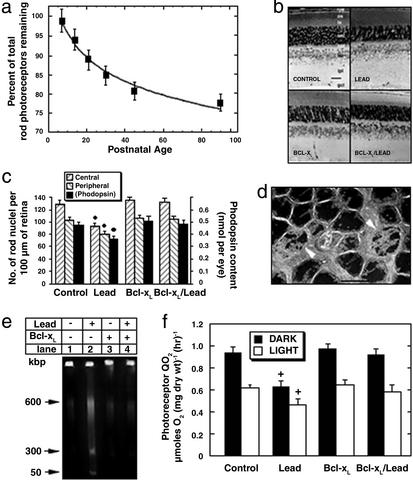
Figure 2.
Bcl-xL overexpression prevents lead-induced rod-selective apoptosis and decreased rod mitochondrial respiration. (a) Kinetics of rod degeneration in developmentally lead-exposed mice. The percent of rods remaining in lead-exposed mice, relative to age-matched controls, was determined from rod count data. The best fit, from nonlinear regression analysis, reveals an exponential decline in the number of rods [y = 116.3 − 20.5 log(x); r = 0.99]. (b) Representative central retinal sections from PN90 CONTROL, Bcl-xL, lead-exposed (LEAD), and Bcl-xL lead-exposed (Bcl-xL/LEAD) mice. (Bar = 25 μm.) (c) Bcl-xL blocks lead-induced rod-selective apoptosis. For PN90 mice, the number of rods ± SEM in central and peripheral retinal regions were counted, and rhodopsin content ± SEM was determined. +, P < 0.05. (d) Electron micrograph of apoptotic rod nuclei (white arrowheads) from a PN21 lead-exposed mouse. (Bar = 5 μm.) (e) Bcl-xL blocks genomic DNA fragmentation in retinas of lead-exposed mice. A representative FIGE gel shows results from PN21 mice. (f) Bcl-xL overexpression prevents lead-induced decrease in QOPR. QOPR was measured in pairs of PN25 dark-adapted and rod saturating light-adapted whole retinas from control, lead, Bcl-xL, and Bcl-xL/lead mice. Values represent the mean ± SEM. +, P < 0.05.
In control mice, immunostaining revealed that Bcl-xL expression was low in all retinal layers (Fig. 1b Left). In transgenic mice, Bcl-xL was overexpressed exclusively in photoreceptors. The highest expression occurred in ISs (Fig. 1b Right), revealing the mitochondrial localization of Bcl-xL. Bcl-xL overexpression in rods, unlike Bcl-2, produced no retinal pathology (Fig. 2b).
Rod Photoreceptor Loss in Lead-Exposed Mice Is Inhibited by Bcl-xL Overexpression.
Low-level lead exposure during development produced a progressive rod-selective degeneration characterized by an exponential decline of rods into adulthood (Fig. 2a). Rod loss was ≈7-fold faster during than after lead exposure even though retinal [Pb] and [Ca] remained elevated (see below). Adult mice (PN90) exposed to lead during development lost one to two rows of rod nuclei and had increased spacing between remaining rod nuclei (Fig. 2b). This loss exhibited a central-to-peripheral gradient such that 27% and 22% of rods in the inferior central and peripheral retina died by apoptosis, and overall 22% of the rods were lost (Fig. 2c). This finding was confirmed by a 24% decrease in rhodopsin content per eye (Fig. 2c). Lead exposure had no effect on cone viability, as mean ± SEM cone counts were not different between groups (3.4 ± 0.4 per 100 μm). Adult Bcl-xL/lead mice exhibited no loss of rods or decrease in rhodopsin content (Fig. 2 b and c) or loss of rods at PN180 (data not shown), demonstrating complete and long-term protection by Bcl-xL overexpression. These results establish the usefulness of this model of progressive rod-selective apoptosis for examining the mechanisms of lead-induced apoptosis and protection by Bcl-xL overexpression in rods.
Bcl-xL Overexpression Blocks Rod Apoptosis and Decreased QOPR, but Not Ca2+ Overload.
Chromatin condensation, high molecular weight DNA fragmentation, and caspase activation are hallmarks of apoptosis (22, 36). EM studies showed the presence of apoptotic rod nuclei in retinas of PN21 lead-exposed mice (Fig. 2d). FIGE revealed that retinas from PN21 lead-exposed mice had a marked increase in 600-, 300-, and 50-kbp DNA fragments (Fig. 2e). These DNA fragmentation and EM results are indicative of apoptotic cell death (36). Bcl-xL overexpression completely blocked the lead-induced rod apoptosis as illustrated by rod cell counts and FIGE (Fig. 2 c and e), suggesting a mitochondrial site of action of lead.
Therefore we measured QOPR in PN25 retinas. In retinas isolated from control and Bcl-xL mice, the dark-adapted QOPR was ≈1 μmol O2 mg dry wt−1⋅hr−1, and it decreased ≈35% during light adaptation (Fig. 2f). Relative to dark- and light-adapted controls, QOPR from lead-exposed mice decreased 34% and 24%, respectively. In lead-exposed mice, Bcl-xL overexpression maintained normal QOPR (Fig. 2f). Similar results were obtained in PN90 mice (data not shown).
Ca2+ is higher in rods during dark than light adaptation, and it produces concentration-dependent decreases in QOPR and increases in rod apoptosis (3, 7, 12, 28). This observation suggests that increased Ca2+ contributes to the retinotoxic effects in lead-exposed mice. The mean ± SEM [Ca] in control rod OS-IS was 23.1 ± 1.6 ppm, which was not different in Bcl-xL transgenics. In PN25 lead-exposed mice, rod [Ca] was increased 37 ± 7% and this was not prevented by Bcl-xL overexpression (39 ± 9%), suggesting that Bcl-xL overexpression does not alter total rod [Ca].
Bcl-xL Overexpression Prevents an Increase in Rod Mitochondrial Contact Sites.
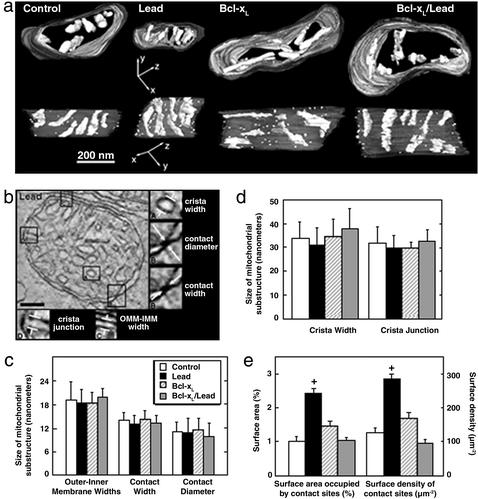
Transmission EM and EM tomography were used to determine whether lead exposure produced ultrastructural or substructural alterations and/or cristae remodeling in PN21 rod mitochondria (Fig. 3). Rod mitochondria displayed an orthodox conformation that was uniform between groups with numerous pleomorphic cristae in an electron-dense matrix (Fig. 3a Upper). There was no physical disruption of the OMM or matrix swelling from lead-exposed mice (Fig. 3 b–d). These observations are supported by detailed analyses of mitochondrial substructures showing no differences between treatment groups for cristae widths, cristae junction diameters, OMM-IMM widths, CS widths, or CS diameters (Fig. 3 c and d). Moreover, the mean ± SD cristae/mitochondrial volume (0.17 ± 0.01), cristae/mitochondrial surface area (1.66 ± 0.37) and cristae segments/unit volume (15.70 ± 2.44) from lead-exposed retinas were not different from control values, indicating no subtle transitions involving cristae fission and tubularization (37).
Figure 3.
Bcl-xL blocks the lead-induced increase in rod mitochondrial contact sites. (a) Three-dimensional reconstructions of rod IS mitochondria imaged in situ from PN21 control, Bcl-xL, lead, and Bcl-xL/lead. (Bar = 200 nm.) (Upper) Surface-rendered depictions of top views after volumes were segmented by contouring the regions bounded by outer (dark gray), inner (light gray), and cristae (white) membranes. Distinct cristae were segmented separately to examine membrane morphology and connectivity. Some of the rendered cristae were omitted for clarity. (Lower) Perpendicular views to those above. Peripheral membranes were made translucent to fully visualize the cristae. Most of the cristae are tubular. Many cristae branch and often their segments are connected by restricted openings. The spatial distribution of CSs is shown as white spheres. (b) Morphological measurements conducted on the reconstructions of rod mitochondria. The large panel shows a 2-nm-thick slice through the reconstructed volume of a representative wild-type lead (LEAD) mitochondrion showing no OMM or IMM breaks or matrix swelling. (Bar = 100 nm.) Smaller panels show illustrations of five classes of substructural measurements obtained. Boxed areas in the large panel are magnified ×2. (c and d) Mean ± SD of measurements highlighted in b. (e) Contact site surface area and surface density measurements. Mean ± SEM measurements of surface area occupied by CSs as a percentage of OMM surface area and surface density of CSs (number per unit surface area of OMM) were made on segmented volumes by using the program XDEND. +, P < 0.05.
Mitochondrial CSs play a fundamental role in energy metabolism, protein import, and presumably apoptosis (18, 20, 21, 29, 38–40). Resectioning of tomographic volumes along various axes confirmed the punctate nature of these CSs (Fig. 3a Lower) and supports the idea that CSs are relatively uniform in different mammalian tissues (29, 41). The 3D distribution of CSs was mapped by using volume segmentation (29). Surface-rendered volumes were displayed and rotated to study the 3D relationships of CSs (Fig. 3 a and b and http://ncmir.ucsd.edu/∼perkins/fox). Two novel results were obtained. First, the CS surface density or number, and surface area were increased ≈2.5-fold in apoptotic rod mitochondria of lead-exposed mice (Fig. 3e). Second, Bcl-xL overexpression completely prevented the lead-induced increase in CSs such that mitochondria in Bcl-xL/Lead mice appeared similar to controls.
Bcl-xL Overexpression Blocks Bax Translocation and Activation of the Cyt c–Caspase-3 Cascade.
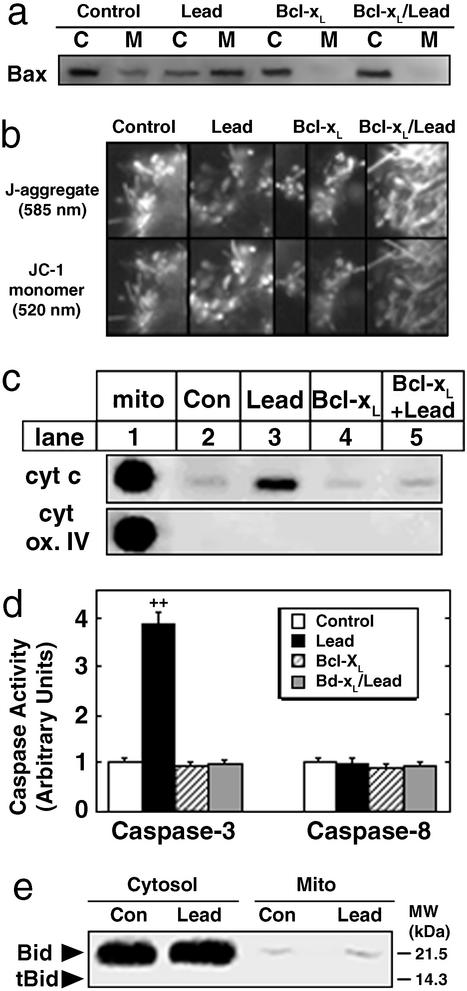
Next, we determined the effects of lead exposure in PN21 mice, with and without Bcl-xL overexpression, on Bax subcellular localization, rod ΔΨm, Cyt c and MDH release, caspase-3 and -8 activity, and Bid cleavage. Developmental lead exposure resulted in the translocation of Bax from the cytosol, where it normally resides (17, 19, 20) in control and Bcl-xL retinas, to the mitochondria. Bcl-xL overexpression prevented this lead-induced translocation (Fig. 4a). Studies with JC-1 revealed that rod mitochondria in control and Bcl-xL retinas had higher ΔΨm, evidenced by the predominance of J-aggregates and minimal presence of JC-1 monomers, compared with a significant proportion of rod mitochondria from lead-exposed mice, evidenced by a decrease in J-aggregates and an increase in JC-1 monomer (Fig. 4b). Bcl-xL overexpression prevented the lead-induced decrease in ΔΨm (Fig. 4b). Coincident with the decreased ΔΨm in lead-exposed rods, 15–20% of the Cyt c from apoptotic rods was released (Fig. 4c) and retinal caspase-3 activity increased 4-fold (Fig. 4d). Bcl-xL overexpression completely blocked all apoptotic events. Lead-induced rod apoptosis occurred in the absence of caspase-8 activation (Fig. 4d) or Bid (p22) cleavage (Fig. 4e), showing that tBid (p15) is not involved in lead-induced Bax translocation or Cyt c release. MDH specific activity ± SEM in control retinas was 1,451 ± 126 nmol mg protein−1⋅min−1 and this partitioned with a 2:1 ratio in the supernatant (68 ± 4%) and mitochondria (33 ± 3%). These values were not significantly different in the other groups (data not shown), indicating that matrix MDH was not released into the cytoplasm during lead exposure.
Figure 4.
Bcl-xL blocks the lead-induced Bax translocation and Cyt c-caspase-3 cascade in rods. (a) A representative immunoblot shows that Bcl-xL blocks lead-induced Bax translocation from cytosol (C) to mitochondria (M) in PN21 retinas of lead-exposed mice. (b) Bcl-xL blocks the lead-induced decrease in rod ΔΨm. ΔΨm was measured in intact rods from PN21 mice by using the JC-1 dye. Mitochondria with higher ΔΨm exhibited J-aggregates, whereas mitochondria with lower ΔΨm were viewed as JC-1 monomers. (c) A representative immunoblot shows that Bcl-xL blocks lead-induced mitochondrial release of Cyt c in PN21 retinas of lead-exposed mice. Mitochondrial fraction (mito) and cytochrome oxidase (cyt ox) IV were controls as described (7). (d) Bcl-xL blocks lead-induced caspase-3 activity (mean ± SEM) in retinas of PN21 lead-exposed mice. ++, P < 0.02. (e) A representative immunoblot of cytosolic and mitochondrial fractions (mito) shows that Bid (p22) was not cleaved to p15 (tBid) in PN21 retinas of lead-exposed mice.
Discussion
During developmental lead exposure, rod photoreceptor [Pb] and [Ca] slowly increase and the rate of rod apoptosis correspondingly increases (refs. 5 and 12; Fig. 2a) as developing rods appear more sensitive to Ca2+ and Pb2+ than mature neurons (5, 8). Similar kinetics of rod degeneration are seen in patients with inherited photoreceptor degenerations (10). The maximal increase of rod [Ca] in lead-exposed mice was 40%, though Ca2+ and Pb2+ act additively to increase rod-selective apoptosis (7). The elevated rod Ca2+ and/or Pb2+ in lead-exposed mice may underlie our observed increase in rod mitochondrial contact sites. This idea is consistent with reports showing that incubation of isolated hearts, brain mitochondria, or liver mitochondria in buffers with elevated Ca2+ increases the formation (number) of CSs (42–44). Similar to the action of overexpressed Bcl-2 in neurons and other cells with low-to-moderate Ca2+ overload (45–47), overexpressed Bcl-xL in rods may help block apoptosis in lead-exposed rods by increasing mitochondrial Ca2+ uptake capacity and preventing a Ca2+-induced loss of ΔΨm. In most models of retinal degeneration, the rod Ca2+ overload is so rapid and massive (≥200%) that apoptosis occurs quickly and Bcl-2 overexpression does not protect (4, 10, 12, 33, 34).
The molecular mechanism of Cyt c release in rod mitochondria from lead-exposed mice is unknown. In lead-exposed mice, Bax is translocated to rod mitochondria, and these mitochondria exhibit a moderate decrease in ΔΨm and release 15–20% of their Cyt c, presumably only from the pool in the intermembrane space (48), but not their matrix MDH. The release of Cyt c occurs in the absence of matrix swelling and OMM rupture. Our results are consistent with findings that (i) transient and nonsynchronous mitochondrial permeability transition pore activation by relatively low concentrations of Ca2+ and/or Bax, or by Bax translocation to the mitochondria after an apoptotic signal induce the release of IMS proteins and the matrix dye calcein without mitochondrial swelling (17, 20, 21, 39, 40, 48–52) and (ii) Bax overexpression in photoreceptors produces rod apoptosis (13). In our lead-exposed mice, Bcl-xL overexpression completely blocked the rod apoptosis, translocation of cytosolic Bax to rod mitochondria, and increase in CSs and activation of the Cyt c-caspase cascade. Therefore, we suggest that overexpressed Bcl-xL in lead-exposed rods complexes with Bax and keeps it from clustering on mitochondria and interacting with different mitochondrial proteins such as VDAC (voltage-dependent anion channel) to release Cyt c and ultimately cause rod apoptosis (17, 19–21, 40). This may be especially important during low-to-moderate Ca2+ overload as Bax, under these conditions, sensitizes the mitochondria to Ca2+-induced mitochondrial permeability transition pore without matrix swelling (50, 51).
In summary, our multiparametric studies demonstrate that environmentally relevant lead exposure in developing mice produces a rod photoreceptor-selective apoptosis that was inhibited by Bcl-xL overexpression. This study presents images of mitochondrial CSs in an in vivo apoptosis model, shows that Bcl-xL overexpression blocks the increased formation of CSs, and demonstrates complete and apparently permanent protection of rod structure and function by Bcl-xL overexpression. These findings have relevance for therapies in a wide variety of retinal and neuronal degenerations where Ca2+ overload, lead exposure, and/or mitochondrial dysfunction occur slowly and continuously over time (2–12, 24, 39, 47, 48).
Acknowledgments
We thank Ms. Yvonne Blocker and Dr. Rick Lawrence for technical assistance, and Drs. David Papermaster and Jolene Windle for the original Bcl-xL transgenic mice. This work was funded by National Institutes of Health Grants ES03183, T35EY07088, EY06891, and EY09213 and a University of Houston Program to Enhance External Research Grant. Part of this work was performed at the National Center for Microscopy and Imaging Research supported by National Institutes of Health Grants RR04050, NS14718, and ES10337.
Abbreviations
- CS
contact site
- Cyt c
cytochrome c
- EM
electron microscopy
- FIGE
field inversion gel electrophoresis
- IMM
inner mitochondrial membrane
- IS
inner segment
- IRBP
interphotoreceptor retinoid binding protein
- JC-1
5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethylbenzimidazolycarbo-cyanine
- MDH
malate dehydrogenase
- ΔΨm
mitochondrial membrane potential
- OMM
outer mitochondrial membrane
- PNn
postnatal day n
- OS
outer segment
- QOPR
rod photoreceptor oxygen consumption
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Thirkill C E, Roth A M, Keltner J L. Arch Ophthalmol. 1987;105:372–375. doi: 10.1001/archopht.1987.01060030092033. [DOI] [PubMed] [Google Scholar]
- 2.Fox D A, Chu L W. Exp Eye Res. 1988;46:613–625. doi: 10.1016/s0014-4835(88)80017-4. [DOI] [PubMed] [Google Scholar]
- 3.Shahinfar S, Edward D P, Tso M O. Curr Eye Res. 1991;10:47–59. doi: 10.3109/02713689109007610. [DOI] [PubMed] [Google Scholar]
- 4.Reme C E, Grimm C, Hafezi F, Marti A, Wenzel A. Prog Retin Eye Res. 1998;17:443–464. doi: 10.1016/s1350-9462(98)00009-3. [DOI] [PubMed] [Google Scholar]
- 5.Fox D A, Campbell M L, Blocker Y S. Neurotoxicology. 1997;18:645–664. [PubMed] [Google Scholar]
- 6.van Soest S, Westerveld A, de Jong P T, Bleeker-Wagemakers E M, Bergen A A. Surv Ophthalmol. 1999;43:321–334. doi: 10.1016/s0039-6257(98)00046-0. [DOI] [PubMed] [Google Scholar]
- 7.He L, Poblenz A T, Medrano C J, Fox D A. J Biol Chem. 2000;275:12175–12184. doi: 10.1074/jbc.275.16.12175. [DOI] [PubMed] [Google Scholar]
- 8.Fox D A, Boyes W K. In: Casarett & Doull's Toxicology: The Science of Poisons. 6th Ed. Klaassen C D, editor. New York: McGraw–Hill; 2001. pp. 565–596. [Google Scholar]
- 9.Rothenberg S J, Schnaas L, Salgado-Valladares M, Casanueva E, Geller A M, Hudnell H K, Fox D A. Invest Ophthalmol Vis Sci. 2002;43:2036–2044. [PubMed] [Google Scholar]
- 10.Clarke G, Collins R A, Leavitt B R, Andrews D F, Hayden M R, Lumsden C J, McInnes R R. Nature. 2000;406:195–199. doi: 10.1038/35018098. [DOI] [PubMed] [Google Scholar]
- 11.Lemasters J J, Nieminen A L, Qian T, Trost L C, Elmore S P, Nishimura Y, Crowe R A, Cascio W E, Bradham C A, Brenner D A, et al. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 12.Fox D A, Poblenz A T, He L. Ann NY Acad Sci. 1999;893:282–285. doi: 10.1111/j.1749-6632.1999.tb07837.x. [DOI] [PubMed] [Google Scholar]
- 13.Eversole-Cire P, Chen J, Simon M I. Invest Ophthalmol Vis Sci. 2002;43:1636–1644. [PubMed] [Google Scholar]
- 14.Martinou J C, Dubois-Dauphin M, Staple J K, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, et al. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 15.Cheng E H, Wei M C, Weiler S, Flavell R A, Mak T W, Lindsten T, Korsmeyer S J. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 16.Lindsten T, Ross A J, King A, Zong W X, Rathmell J C, Shiels H A, Ulrich E, Waymire K G, Mahar P, Frauwirth K, et al. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korsmeyer S J, Wei M C, Saito M, Weiler S, Oh K J, Schlesinger P H. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 18.Lutter M, Perkins G A, Wang X. BMC Cell Biol. 2001;2:22. doi: 10.1186/1471-2121-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nechushtan A, Smith C L, Lamensdorf I, Yoon S H, Youle R J. J Cell Biol. 2001;153:1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capano M, Crompton M. Biochem J. 2002;367:169–178. doi: 10.1042/BJ20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vyssokikh M Y, Zorova l, Zorov D, Heimlich G, Jurgenmeier J M, Brdiczka D. Mol Biol Rep. 2002;29:93–96. doi: 10.1023/a:1020383108620. [DOI] [PubMed] [Google Scholar]
- 22.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 23.Hentunen T A, Reddy S V, Boyce B F, Devlin R, Park H R, Chung H, Selander K S, Dallas M, Kurihara N, Galson D L, et al. J Clin Invest. 1998;102:88–97. doi: 10.1172/JCI2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medrano C J, Fox D A. Toxicol Appl Pharmacol. 1994;125:309–321. doi: 10.1006/taap.1994.1077. [DOI] [PubMed] [Google Scholar]
- 25.Shulman L M, Fox D A. Proc Natl Acad Sci USA. 1996;93:8034–8039. doi: 10.1073/pnas.93.15.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajek P, Villani G, Attardi G. J Biol Chem. 2001;276:606–615. doi: 10.1074/jbc.M007871200. [DOI] [PubMed] [Google Scholar]
- 27.Kueng-Hitz N, Grimm C, Lansel N, Hafezi F, He L, Fox D A, Reme C E, Niemeyer G, Wenzel A. Invest Ophthalmol Vis Sci. 2000;41:909–916. [PubMed] [Google Scholar]
- 28.Medrano C J, Fox D A. Exp Eye Res. 1995;61:273–284. doi: 10.1016/s0014-4835(05)80122-8. [DOI] [PubMed] [Google Scholar]
- 29.Perkins G, Renken C, Martone M E, Young S J, Ellisman M, Frey T. J Struct Biol. 1997;119:260–272. doi: 10.1006/jsbi.1997.3885. [DOI] [PubMed] [Google Scholar]
- 30.Perkins G, Renken C, Song J Y, Frey T G, Young S J, Lamont S, Martone M E, Lindsey S, Ellisman M H. J Struct Biol. 1997;120:219–227. doi: 10.1006/jsbi.1997.3920. [DOI] [PubMed] [Google Scholar]
- 31.Mastronarde D N J. J Struct Biol. 1997;120:343–352. doi: 10.1006/jsbi.1997.3919. [DOI] [PubMed] [Google Scholar]
- 32.Darley-Usmar V M, Rickwood D, Wilson M T. Mitochondria: A Practical Approach. Oxford: IRL Press; 1987. pp. 79–112. [Google Scholar]
- 33.Eversole-Cire P, Concepcion F A, Simon M I, Takayama S, Reed J C, Chen J. Invest Ophthalmol Vis Sci. 2000;41:1953–1961. [PubMed] [Google Scholar]
- 34.Quiambao A B, Tan E, Chang S, Komori N, Naash M I, Peachey N S, Matsumoto H, Ucker D S, Al Ubaidi M R. Exp Eye Res. 2001;73:711–721. doi: 10.1006/exer.2001.1083. [DOI] [PubMed] [Google Scholar]
- 35.Bowes C, van Veen T, Farber D B. Exp Eye Res. 1988;47:369–390. doi: 10.1016/0014-4835(88)90049-8. [DOI] [PubMed] [Google Scholar]
- 36.Brown D G, Sun X M, Cohen G M. J Biol Chem. 1993;268:3037–3039. [PubMed] [Google Scholar]
- 37.Mannella C A, Pfeiffer D R, Bradshaw P C, Morau I I, Slepchenko B, Loew L M, Hsieh C, Buttle K, Marko M. IUBMB Life. 2001;52:93–100. doi: 10.1080/15216540152845885. [DOI] [PubMed] [Google Scholar]
- 38.Reichert A S, Neupert W. Biochim Biophys Acta. 2002;1592:41–49. doi: 10.1016/s0167-4889(02)00263-x. [DOI] [PubMed] [Google Scholar]
- 39.Bernardi P. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 40.Doran E, Halestrap A P. Biochem J. 2000;348:343–350. [PMC free article] [PubMed] [Google Scholar]
- 41.Perkins G A, Frey T G. Micron. 2000;31:97–111. doi: 10.1016/s0968-4328(99)00065-7. [DOI] [PubMed] [Google Scholar]
- 42.Van Venetie R, Verkleij A J. Biochim Biophys Acta. 1982;692:397–405. doi: 10.1016/0005-2736(82)90390-x. [DOI] [PubMed] [Google Scholar]
- 43.Sandri G, Siagri M, Panfli E. Cell Calcium. 1988;9:159–165. doi: 10.1016/0143-4160(88)90020-6. [DOI] [PubMed] [Google Scholar]
- 44.Bakker A, Bernaert I, De Bie M, Ravingerova T, Ziegelhoffer A, Van Belle H, Jacob W. Biochim Biophys Acta. 1994;1224:583–588. doi: 10.1016/0167-4889(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 45.Murphy A N, Bredesen D E, Cortopassi G, Wang E, Fiskum G. Proc Natl Acad Sci USA. 1996;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu L, Ling S, Yu X D, Venkatesh L K, Subramanian T, Chinnadurai G, Kuo T H. J Biol Chem. 1999;274:33267–33273. doi: 10.1074/jbc.274.47.33267. [DOI] [PubMed] [Google Scholar]
- 47.Kruman I I, Mattson M P. J Neurochem. 1999;72:529–540. doi: 10.1046/j.1471-4159.1999.0720529.x. [DOI] [PubMed] [Google Scholar]
- 48.Bernardi P, Petronilli V, Di Lisa F, Forte M. Trends Biochem Sci. 2001;26:112–117. doi: 10.1016/s0968-0004(00)01745-x. [DOI] [PubMed] [Google Scholar]
- 49.Andreyev A Y, Fahy B, Fiskum G. FEBS Lett. 1998;439:373–376. doi: 10.1016/s0014-5793(98)01394-5. [DOI] [PubMed] [Google Scholar]
- 50.Pastorino J G, Tafani M, Rothman R J, Marcinkeviciute A, Hoek J B, Farber J L, Marcineviciute A. J Biol Chem. 1999;274:31734–31739. doi: 10.1074/jbc.274.44.31734. [DOI] [PubMed] [Google Scholar]
- 51.Gogvadze V, Robertson J D, Zhivotovsky B, Orrenius S. J Biol Chem. 2001;276:19066–19071. doi: 10.1074/jbc.M100614200. [DOI] [PubMed] [Google Scholar]
- 52.Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes S A, Mannella C A, Korsmeyer S J. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]