Abstract
Pituitary tumor development involves clonal expansion stimulated by hormones and growth factors/cytokines. Using mRNA differential display, we found that the bone morphogenetic protein (BMP) inhibitor noggin is down-regulated in prolactinomas from dopamine D2-receptor-deficient mice. BMP-4 is overexpressed in prolactinomas taken from dopamine D2-receptor-deficient female mice, but expression of the highly homologous BMP-2 does not differ in normal pituitary tissue and prolactinomas. BMP-4 is overexpressed in other prolactinoma models, including estradiol-induced rat prolactinomas and human prolactinomas, compared with normal tissue and other pituitary adenoma types (Western blot analysis of 48 tumors). BMP-4 stimulates, and noggin blocks, cell proliferation and the expression of c-Myc in human prolactinomas, whereas BMP-4 has no action in other human pituitary tumors. GH3 cells stably transfected with a dominant negative of Smad4 (Smad4dn; a BMP signal cotransducer) or noggin have reduced tumorigenicity in nude mice. Tumor growth recovered in vivo when the Smad4dn expression was lost, proving that BMP-4/Smad4 are involved in tumor development in vivo. BMP-4 and estrogens act through overlapping intracellular signaling mechanisms on GH3 cell proliferation and c-myc expression: they had additive effects at low concentrations but not at saturating doses, and their action was inhibited by blocking either pathway with the reciprocal antagonist (i.e., BMP-4 with ICI 182780 or 17β-estradiol with Smad4dn). Furthermore, coimmunoprecipitation studies demonstrate that under BMP-4 stimulation Smad4 and Smad1 physically interact with the estrogen receptor. This previously undescribed prolactinoma pathogenesis mechanism may participate in tumorigenicity in other cells where estrogens and the type β transforming growth factor family have important roles.
Keywords: signal transduction‖pituitary neoplasms
There is little doubt that estrogens and growth factors are involved in the control of lactotroph cell proliferation. In vitro, as well as clinical, evidence demonstrates the tumorigenic action of estrogen in prolactinomas. Thus, the number of lactotroph cells increases during pregnancy (1). Prolactinomas occur more frequently in women and increase in size during pregnancy or estrogen treatment (2, 3), and, at least in human prolactinomas, estrogen receptor (ER) expression is positively related to size (4). Dominant-negative ER inhibits growth of lactotroph cells in nude mice (5). In female rats, estrogens promote the development of experimental prolactinomas (6, 7), whereas in dopamine D2-receptor (D2R)-deficient mice (D2R−/−), a new animal model for prolactinomas, these grow spontaneously only in females (8, 9). Estrogens regulate somatostatin receptor expression in prolactin (PRL)-secreting pituitary tumor cells (10). By using GH3 rat tumor cells and other models, it has been shown that estrogen-induced pituitary tumor-derived transforming gene (PTTG) and fibroblast growth factor are involved in prolactinoma pathogenesis (7).
Pituitary cells produce cytokines and express their receptors. This constitutes the basis for the paracrine and autocrine action of cytokines in the control of hormone production and cell proliferation in the pituitary (11–15). Growth factors and hypothalamic factors also play a part in the molecular and cellular mechanisms that lead to prolactinoma development (11–13). Bone morphogenetic proteins (BMPs), as well as other members of the type β transforming growth factor (TGFβ) family, transduce signals through Smad4, a signal cotransducer (16–18). Smad4, in turn, regulates c-myc, a protooncogene that controls cell cycle and mediates the effects of TGFβ on cell proliferation (19, 20). We report here a previously undescribed mechanism for prolactinoma growth that involves BMP-4, Smad4, and estrogens.
Methods
Animal Housing and Experiments.
Animals were housed under standard conditions. Anterior pituitaries from female D2R−/−, heterozygous (D2R+/−), and C57 normal control (D2R+/+) mice were used (8, 9). Primary prolactinomas were induced in Sprague–Dawley female rats by weekly i.m. injections of 40 mg of 17β-estradiol, carried out for 4 weeks as described (21). N:NIH (S)-nu nude mice were injected s.c. with 1 × 106 cells, and tumor growth was determined as described (22). All protocols were approved by the Ethical Committee on Animal Care and Use, University of Buenos Aires, Argentina.
Differential Display.
A pituitary tumor from an 18-month-old female D2R−/− was used for differential display (8, 9). Normal pituitary tissue from D2R+/+ was used for comparison. Total RNA was isolated as described (23). mRNA differential display was performed with an RNAimage kit (Gene Hunter, Nashville, TN).
PCR.
Total RNA was extracted from a pituitary tumor from a female D2R−/− and from pituitaries from D2R+/− and D2R+/+ as described (23). RT-PCR was performed under restrictive conditions by using only 25 amplification cycles (1 min, 94°C; 1 min, 55°C; 1 min, 72°C) to obtain band intensities proportional to the RNA amount (24). Actin was amplified from the same samples under the same conditions, as an internal control. The primer sequences were: noggin, CGGGGACGCGGGACGAAGAG, CGGTCCTCTGGGGGCGAAGT; actin, ACGGGGTCACCCACACTGTGC, CTAGAAGCATTTGCGGTGGACGATG.
Plasmids.
The Smad4 dominant negative (Smad4dn) expression vector consists of a truncated DPC4 (Smad4) cDNA (1–514) fused to FLAG expressed under the control of the cytomegalovirus (CMV) promoter (25). The noggin expression vector contains 1 kb of the mouse noggin cDNA driven by the translation elongation factor (EF)-1α promoter (26).
Cell Culture and Stable Clones.
All materials were from Invitrogen unless otherwise stated. GH3 cells were cultured in DMEM with 10% FCS as described (27). Treatments with 17β-estradiol were performed in phenol red-free DMEM with stripped FCS (28). GH3 cells were stably transfected with noggin or Smad4dn expression vectors using Lipofectamine and selected with 600 ng/ml G418. GH3–noggin clones were tested for noggin expression by Northern blot analysis (26). GH3–Smad4dn clones were checked by Western blot analysis with anti-FLAG antibodies (Chemicon). Three additional stable clones for each construct had results similar to the one shown. Cells were treated with 10, 50, 100, or 200 ng/ml BMP-4; 0.1 or 1 μg/ml noggin (both from R & D Systems); 1, 10, or 100 nM 17β-estradiol; 1 μM tamoxifen (both from Sigma); 10 ng/ml platelet-derived growth factor (PDGF; Roche Molecular Biochemicals); 10 or 100 nM thyrotropin-releasing hormone (TRH; Bachem); or 1, 10, or 100 nM or 1 μM ICI 182780 (Tocris Cookson, Ballwin, MO).
Tumors.
Pituitary tumors were obtained from patients with hyperprolactinemia, acromegaly, Cushing's disease, or clinical diagnosis of nonfunctioning pituitary adenomas. Hormone production was confirmed by immunohistochemistry, and tumor samples were treated as explants, as described (23, 29).
Cell Proliferation and PRL Measurement.
A WST-1 assay (Roche Molecular Biochemicals) was used to measure cell proliferation as described (30). Staining with acridine orange and ethidium bromide was used to rule out toxic effects. Rat PRL was measured by RIA as described (27).
Coimmunoprecipitation.
GH3 cells were treated as described for 1 h, and then cell lysates were immunoprecipitated with anti-Smad4, anti-ERα, or anti-ERβ. Protein A Sepharose (Amersham Pharmacia Biotech) was used as described (31). Experiments were performed by using agarose-conjugated anti-Smad4 (Santa Cruz Biotechnology) with similar results.
Western Blot Analysis.
Cells were treated as described; lysates were analyzed by PAGE and blotted by using standard procedures, and anti-BMP-4, anti-Smad1, anti-Smad2, anti-Smad4, anti-ERα, anti-ERβ, anti-c-Myc (Santa Cruz Biotechnology), or anti-FLAG (Chemicon) was used. Anti-β-actin (Sigma) was used routinely as loading control.
Statistical Analysis.
Results are expressed as mean ± SE. Differences were assessed by one-way ANOVA in combination with Scheffé's test.
Results
Overexpression of BMP-4 in Prolactinomas.
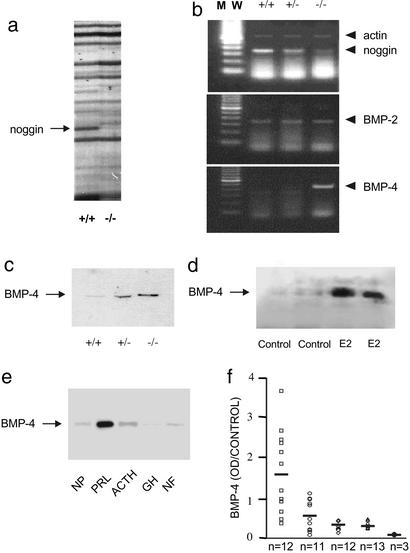
We used mRNA differential display in female D2R−/− mice to compare the gene expression pattern in normal anterior pituitaries and prolactinomas. A band present only in the normal pituitary tissue was identified as noggin, a specific inhibitor of BMP action that does not inhibit other members of the TGFβ family (16, 32) (Fig. 1a). Comparative RT-PCR confirmed the differential expression of noggin (Fig. 1b). BMP-4 was overexpressed in prolactinomas taken from female D2R−/− mice (Fig. 1 b and c). Expression levels of the highly homologous BMP-2, however, did not differ between normal pituitary tissue and prolactinomas (Fig. 1b). An equivalent overexpression of BMP-4 also was found in prolactinomas from female Sprague–Dawley rats, induced by 17β-estradiol treatment (Fig. 1d). Given our finding of BMP-4 overexpression in different models of pituitary prolactinomas, we next assessed BMP-4 expression in 48 human pituitary tumors. BMP-4 also was significantly overexpressed in human prolactinomas, compared with normal human pituitaries and other pituitary adenoma types (Fig. 1 e and f). The protein expression of Smad4 was detected in all models of prolactinomas, i.e., D2R−/− mice, 17β-estradiol-treated rats, and human prolactinomas (data not shown).
Figure 1.
Differential expression of the BMP-4 system in prolactinomas. (a) mRNA differential display of pituitaries from D2R+/+ (control) mice and D2R−/− mice with prolactinoma. Arrow indicates the band identified as noggin. These results represent four independent amplifications. (b) Comparative RT-PCR was performed to detect noggin, BMP-2, and BMP-4 in RNA extracted from pituitaries taken from D2R+/+, D2R+/−, or D2R−/− mice. Actin amplification was performed in the same reaction tubes under suboptimal conditions as an internal standard. These results represent three independent RT-PCR reactions. (c) Differential expression of BMP-4 protein was confirmed by Western blot analysis in protein extracts from normal pituitaries taken from D2R+/+ (control) mice and from D2R+/− mice without tumors, and from prolactinomas taken from D2R−/− mice. Equal loading was assessed by β-actin detection. These results represent two independent experiments with similar results. (d) BMP-4 was measured by Western blot analysis in pituitaries from four female Sprague–Dawley rats treated with vehicle (control; plasma PRL, 38.65 ± 0.45 ng/ml) or 200 mg/ml 17β-estradiol (E2; plasma PRL, 446.5 ± 42.5 ng/ml) as detailed in Methods. Equal loading was assessed by β-actin detection. (e) BMP-4 was examined by Western blot analysis in 51 protein homogenates, each obtained from one of 51 individual samples (distributed as shown in f) from normal human pituitary tissue (NP; n = 3); human prolactinomas (PRL; n = 12); human corticotropin (ACTH)-secreting tumors (ACTH; n = 11); human growth hormone (GH)-secreting tumors (GH; n = 12); or clinically inactive pituitary tumors (NF; n = 13); one representative example of each case is shown. Equal loading was assessed by β-actin detection. (f) BMP-4 detections by Western blot from the 51 samples used in e were analyzed by densitometry and normalized by using β-actin values as loading control. ●, normal human pituitary tissue; □, human prolactinomas; ○, human ACTH-secreting tumors; ⋄, human GH-secreting tumors; ▵, clinically inactive pituitary tumors. In many cases, symbols corresponding to individual tumors overlap. BMP-4 levels in human prolactinomas are significantly different (P < 0.01) with respect to all other groups, which show no statistical difference among them (ANOVA with Scheffé's test).
BMP-4 Stimulates Prolactinoma Development.
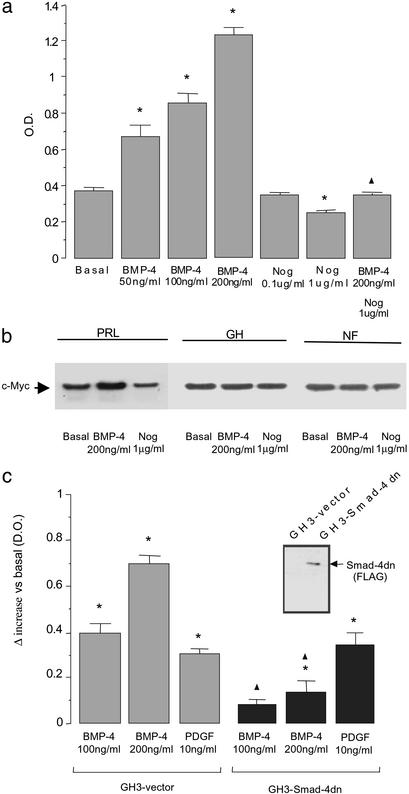
BMP-4 did not significantly affect PRL production in GH3 and in normal rat pituitary cells (data not shown). BMP-4 did lead to a major increase in cell proliferation in GH3 cells; this effect was blocked by noggin (Fig. 2a). We next tested the ability of BMP-4 to stimulate in human tumors the expression of c-Myc (Fig. 2b), an important regulator of cell cycle progression and a target for Smad pathways (19, 20). In human pituitary prolactinoma explants, but not in other human adenoma types, BMP-4 stimulated and noggin inhibited (as they did in GH3 proliferation) c-Myc expression (Fig. 2b).
Figure 2.
BMP-4 stimulates the proliferation of a lactotroph tumor cell line. (a) GH3 cells were treated with BMP-4, noggin (Nog), or their combination for 72 h. Cell proliferation was measured by WST-1 assay. Results represent the mean ± SE of quadruplicates from four independent experiments. *, P < 0.01 with respect to basal values; ▴, P < 0.01 with respect to 200 ng/ml BMP-4 stimulation (ANOVA with Scheffé's test). (b) Tumor explants were treated with BMP-4 or noggin (Nog) for 90 min, and c-Myc expression was analyzed by Western blot analysis as a parameter related to cell proliferation in different types of pituitary tumors (n = 9). PRL, human prolactinoma; GH, human GH-secreting pituitary tumor; NF, clinically inactive pituitary tumor. Equal loading was assessed by β-actin detection. (c) Cell proliferation under BMP-4 treatment was compared between GH3 cells stably transfected with an empty vector and GH3 cells with Smad4dn. Cells were treated for 72 h, and proliferation was measured by WST-1 assay. PDGF stimulation of GH3 cell proliferation was used as a positive control unrelated to BMP signaling. Bars represent the mean ± SE of the differences between the treated and the corresponding basal values of quadruplicates from three independent experiments. *, P < 0.01 with respect to the corresponding basal values (GH3–vector, 0.305 ± 0.015; GH3–Smad4dn, 0.365 ± 0.020); ▴, P < 0.01 comparing GH3–vector and GH3–Smad4dn cells under the same treatment (ANOVA with Scheffé's test). (Inset) Smad4dn (FLAG) expression was checked by Western blot analysis against a FLAG epitope contained in the GH3 cells with Smad4dn, as described in Methods.
To further study tumor formation in nude mice, we produced GH3 clones stably transfected with a Smad4dn (GH3–Smad4dn). Smad4dn blocked the stimulatory effect of BMP-4 on cell proliferation (Fig. 2c). However, the action of PDGF (which is not mediated by Smad4) was not affected (Fig. 2c). Similar results were obtained with clones overexpressing noggin (data not shown).
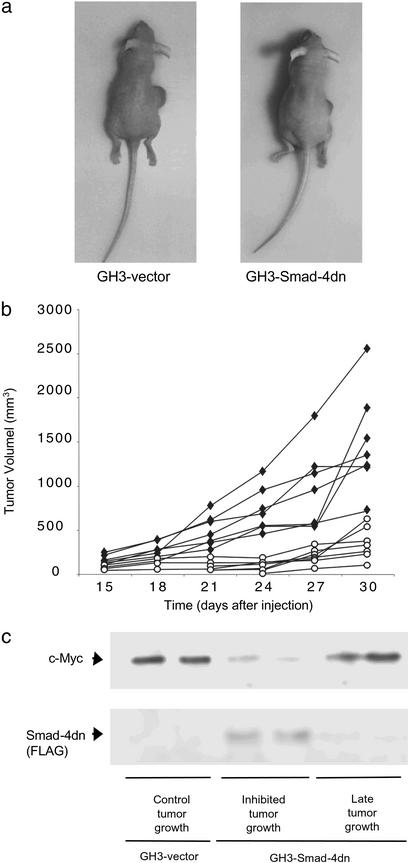
Tumor formation in nude mice with GH3 cells expressing Smad4dn was reduced markedly, demonstrating that the BMP-4 stimulatory pathway plays a role in the development of pituitary tumors in vivo. Cells transfected with an empty vector formed fast-growing, large tumors and had high c-Myc levels, whereas Smad4dn dramatically reduced tumor growth and c-Myc expression (Fig. 3). Similar results were obtained with cells transfected with a noggin expression vector (data not shown). Two tumors produced by Smad4dn cells escaped from the initial Smad4dn inhibition and showed a late increase in tumor size similar to that shown by cells transfected with an empty vector. The molecular analysis of these tumors showed that both had lost in vivo the expression of Smad4dn and had recovered c-Myc expression at levels similar to those of the controls (Fig. 3c), providing direct evidence of the involvement of BMP-4 and Smad4 in tumorigenesis in vivo.
Figure 3.
Smad4dn inhibits tumor growth in vivo. (a) Nude mice were injected s.c. with GH3 cells stably transfected with an empty vector (GH3–vector) or with a vector expressing Smad4dn (GH3–Smad4dn). At 30 days after injection, large tumors formed by GH3–vector cells were observed and compared with the smaller tumors in animals injected with GH3–Smad4dn cells. (b) Tumors of animals injected as indicated in a were detected 15 days after injection, and growth was monitored for a total of 30 days. ♦, GH3–vector; ○, GH3–Smad4dn. These results represent two independent experiments in which three and four animals, respectively, were injected with each cell line. (c) c-Myc and Smad4dn (FLAG) expression was analyzed by Western blot in tumor samples from nude mice injected with GH3 cells stably transfected with an empty vector (control tumor growth) or Smad4dn-expressing GH3 cells (inhibited tumor growth) after 30 days. Two additional tumor samples that escaped from the initial Smad4dn inhibition and presented a late increase in tumor size (late tumor growth) were analyzed for c-Myc and Smad4dn (FLAG) expression by Western blot. Equal loading was assessed by β-actin detection.
Crosstalk Between BMP-4 and Estrogen Signaling.
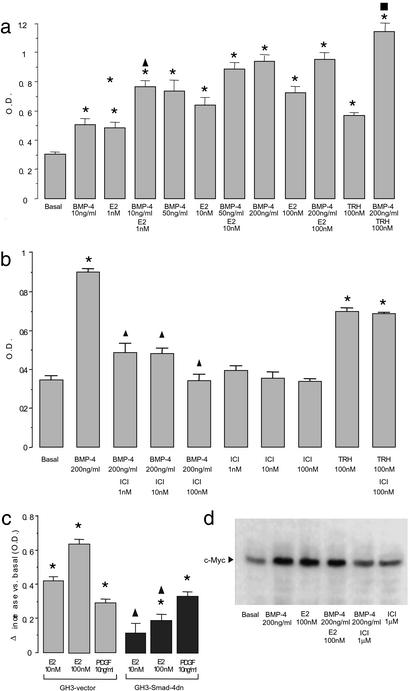
We next studied whether a crosstalk exists between BMP-4 and estrogen signaling. BMP-4 and 17β-estradiol individually increased GH3 cell proliferation; both treatments in combination did not lead to any further increase at saturating doses, but had an additive effect at low concentrations (Fig. 4a). Blocking either pathway (i.e., estrogens or BMP-4) with the reciprocal antagonist (i.e., BMP-4 with ICI 182780 or 17β-estradiol with Smad4dn) resulted in a partial but significant inhibition of its action on cell proliferation (Fig. 4 b and c), confirming that BMP-4 and estrogens act through overlapping intracellular signaling mechanisms. The interaction between BMP-4 and 17β-estradiol was evidenced further by a similar expression pattern of the estrogen and Smad4 target c-Myc (19, 20, 33) (Fig. 4d).
Figure 4.
Crosstalk between BMP-4 and estrogen signaling. (a) GH3 cells were treated with BMP-4, 17β-estradiol (E2), or their combination as indicated. After 72 h, cell proliferation was measured by WST-1 assay. As a control, cells were treated with TRH in combination with BMP-4, which, at the same saturating dose at which it did not interact with estrogen, produced a greater effect than did BMP-4 or TRH individually. *, P < 0.01 compared with basal values; ▴, P < 0.01 compared with 10 ng/ml BMP-4 or 1 nM 17β-estradiol individually; ■, P < 0.01compared with TRH or 200 ng/ml BMP-4 individually (ANOVA with Scheffé's test). (b) GH3 cells were treated with BMP-4, ICI 182780 (ICI), or their combination as indicated. After 72 h, cell proliferation was measured by WST-1 assay. The effect of TRH (used as a control) was not inhibited, indicating the specificity of the effect of ICI 182780. Similar results were obtained with tamoxifen. Bars represent the mean ± SE of quadruplicates from three independent experiments. *, P < 0.01compared with basal values; ▴, P < 0.01 compared with 200 ng/ml BMP-4 (ANOVA with Scheffé's test). (c) 17β-estradiol stimulation of cell proliferation was compared between GH3–vector and GH3–Smad4dn cells. Cells were treated with 17β-estradiol for 72 h, and cell proliferation was measured by WST-1 assay. PDGF stimulation was performed as a positive control of similar responsiveness between cell lines. Bars represent the mean ± SE of the differences between the treated and the corresponding basal values of quadruplicates from three independent experiments. *, P < 0.01 compared with the corresponding basal values (GH3–vector, 0.335 ± 0.011; GH3–Smad4dn, 0.395 ± 0.020); ▴, P < 0.01 between the two cell lines under the same treatment (ANOVA with Scheffé's test). (d) GH3 cells were treated with BMP-4, 17β-estradiol, ICI 182780, or their combination as indicated. After a 1-h treatment, cells were lysed, and the protein extracts were analyzed by Western blot for c-Myc as described in Methods. Equal loading was assessed by β-actin detection. One representative of three independent experiments with similar results is shown.
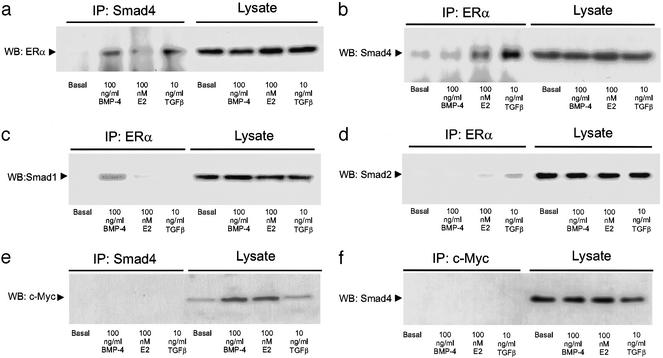
Coimmunoprecipitation studies demonstrate that the ER can physically interact with Smad4 and the specific BMP signal transducer, Smad1. Thus, Smad4 and Smad1, but not Smad2, coimmunoprecipitated with ERα in the presence of BMP-4, whereas, under TGFβ treatment, Smad2 and Smad4, but not Smad1, coimmunoprecipitated with ERα (Fig. 5). Control antibodies did not produce coimmunoprecipitated proteins (Fig. 5 e and f). Similar results were obtained with ERβ (data not shown).
Figure 5.
Smad and ER physical association. GH3 cells were treated with BMP-4, 17β-estradiol (E2), or TGFβ for 1 h. Cell lysates were immunoprecipitated with Protein A Sepharose in combination with the following primary antibodies: (a and e) anti-Smad4, (b–d) anti-ERα, and (f) anti-c-Myc. The immunoprecipitated fractions and the whole lysates were analyzed by Western blot as described in Methods, by using the following antibodies: (a) anti-ERα, (b and f) anti-Smad4, (c) anti-Smad1, (d) anti-Smad2, and (e) anti-c-Myc, which was used as an unrelated control. One representative of four independent experiments with similar results is shown. Similar results were obtained by using anti-Smad4 conjugated with agarose.
Discussion
In this report we show that noggin is down-regulated in D2R−/− mouse prolactinomas and BMP-4 is overexpressed in this and other prolactinoma models, including estradiol-induced rat prolactinomas and human pituitary adenomas, compared with normal tissue and other pituitary adenoma types. In addition, BMP-4 has a strong stimulatory effect on cell proliferation. This system is active both in vitro and in vivo as demonstrated by the inhibition of tumor growth in nude mice by Smad4dn or noggin overexpression. Other members of the TGFβ family have inhibitory effects on prolactinomas (34, 35), and TGFβ inhibits c-Myc expression (19, 36). However, BMP-4 positive growth signaling in the control of PRL-secreting cells overrides the negative effect of TGFβ, because GH3–Smad4dn cells (which do not respond to either BMP-4 or TGFβ) have a blunted growth in nude mice. Both noggin, which is specific for BMP-4, and Smad4dn blocked tumor growth in vivo. Because BMP-4 stimulates prolactinoma growth, whereas TGFβ inhibits it, the results obtained with Smad4dn clones provide even stronger confirmation of the role of BMP-4 in tumor growth. One of the central questions in tumor growth control is how tumor cells escape from the inhibition of cell proliferation by TGFβ family members (37). We propose that prolactinomas, in which BMP-4 stimulates cell proliferation, may be an interesting model to investigate these mechanisms.
BMP-2 and BMP-4 have been shown to play a role in the initial steps of the development of the anterior pituitary (38). For instance, BMP-4 is required for the proliferation of the Rathke's pouch placode, which gives rise to PRL-secreting cells, among others. The overexpression of noggin or a dominant-negative BMP-receptor (BMPR1A) in the anterior pituitary leads to an arrest in the development of PRL-secreting cells (38). This fits in with our results, which introduce the concept of BMP-4 as a positive stimulus for the proliferation of adult PRL-secreting tumor cells.
Our data show that the BMP-4 signal transduction interacts with estrogen and that this interaction is mediated by ERs and Smad4. This interaction may explain the high incidence of prolactinomas in women. BMP-4 effects are partially mediated by ERs, and, conversely, estradiol effects are partially mediated by Smad4, because antiestrogens and Smad4dn partially inhibit the effects of BMP-4 and estradiol, respectively. The fact that antiestrogens partially inhibit BMP-4 effects in prolactinoma cells suggests that the crosstalk between BMP-4 and estrogens could provide a new target for specific antiestrogenic drugs. Moreover, the physical interaction between Smad4 and ER may be present not only in prolactinoma cells but also in others such as breast and bone, in which both estrogens and the TGFβ family play important roles (16, 37, 39).
Previous studies on the signal transduction of TGFβ family members have shown that Smad4 is a common mediator that binds to DNA and regulates gene transcription (40). Smads may interact with other transcription partners (41), and some of the factors that cooperate with Smad to regulate gene transcription have been identified (16, 37, 40). The transcriptional mechanisms of estrogen action have been characterized (42), and interactions between different ERs (α and β) with different ligands, response elements, and nuclear cofactors may explain the tissue specificity of estrogen and antiestrogen effects (42, 43). Smad proteins mediate transcriptional activation or repression depending on their associated partners (44). Here we describe a direct physical interaction between ER, Smad4, and Smad1 (but not Smad2) in association with a specific BMP-4-induced crosstalk mechanism that stimulates cell proliferation. In contrast, TGFβ induced a physical interaction between ER and Smad2 or Smad4, but not Smad1. A similar interaction between ER and TGFβ-induced Smad3 recently has been shown in a reconstituted signaling system (45). These different signaling complexes and associated proteins specifically induced by BMP-4 or TGFβ might provide a basis to explain their different proliferative effects. The rapid induction of c-myc by BMP-4 and estrogens suggest that the c-myc promoter might be a direct target of these ER–Smad complexes. BMP-4, other members of the TGFβ family, and estrogens play significant roles in several tumor types and in bone physiology. We demonstrate for the first time that a BMP-4–Smad–ER molecular regulatory mechanism exists and is of critical importance in a physiologically relevant model. Similar mechanisms may well play a part in the progression of other diseases.
Acknowledgments
We thank J. Massague for the Smad4dn expression vector and K. Monzen for the noggin expression vector; A. Rosenkranz, G. E. Lammel, M. M. Ricca, and M. Papazoglou for technical assistance; and P. Rosenfeld for reviewing the manuscript for English usage. This work was supported by grants from the Volkswagen Foundation (I/76 803), the University of Buenos Aires, the Argentine Health Ministry (Carrillo-Oñativia grant), the Argentine National Research Council, and the Agencia Nacional de Promoción Científica y Tecnológica, Argentina.
Abbreviations
- ER
estrogen receptor
- D2R
dopamine D2 receptor
- PRL
prolactin
- BMP
bone morphogenetic protein
- TGFβ
transforming growth factor β
- Smad4dn
Smad4 dominant negative
- TRH
thyrotropin-releasing hormone
References
- 1.Stefaneanu L, Kovacs K, Lloyd R V, Scheithauer B W, Young W F, Jr, Sano T, Jin L. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62:291–296. doi: 10.1007/BF02899695. [DOI] [PubMed] [Google Scholar]
- 2.Holmgren U, Bergstrand G, Hagenfeldt K, Werner S. Acta Endocrinol. 1986;111:452–459. doi: 10.1530/acta.0.1110452. [DOI] [PubMed] [Google Scholar]
- 3.Garcia M M, Kapcala L P. J Endocrinol Invest. 1995;18:450–455. doi: 10.1007/BF03349744. [DOI] [PubMed] [Google Scholar]
- 4.Stefaneanu L, Kovacs K, Horvath E, Lloyd R V, Buchfelder M, Fahlbusch R, Smyth H. J Clin Endocrinol Metab. 1994;78:83–88. doi: 10.1210/jcem.78.1.8288720. [DOI] [PubMed] [Google Scholar]
- 5.Lee E J, Duan W R, Jakacka M, Gehm B D, Jameson J L. Endocrinology. 2001;142:3756–3763. doi: 10.1210/endo.142.9.8372. [DOI] [PubMed] [Google Scholar]
- 6.Wiklund J, Wertz N, Gorski J. Endocrinology. 1981;109:1700–1707. doi: 10.1210/endo-109-5-1700. [DOI] [PubMed] [Google Scholar]
- 7.Heaney A P, Horwitz G A, Wang Z, Singson R, Melmed S. Nat Med. 1999;5:1317–1321. doi: 10.1038/15275. [DOI] [PubMed] [Google Scholar]
- 8.Kelly M A, Rubinstein M, Asa S L, Zhang G, Saez C, Bunzow J R, Allen R G, Hnasko R, Ben-Jonathan N, Grandy D K, Low M J. Neuron. 1997;19:103–113. doi: 10.1016/s0896-6273(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 9.Asa S L, Kelly M A, Grandy D K, Low M J. Endocrinology. 1999;140:5348–5355. doi: 10.1210/endo.140.11.7118. [DOI] [PubMed] [Google Scholar]
- 10.Visser-Wisselaar H A, Van Uffelen C J, Van Koetsveld P M, Lichtenauer-Kaligis E G, Waaijers A M, Uitterlinden P, Mooy D M, Lamberts S W, Hofland L J. Endocrinology. 1997;138:1180–1189. doi: 10.1210/endo.138.3.5016. [DOI] [PubMed] [Google Scholar]
- 11.Arzt E, Páez-Pereda M, Castro C P, Pagotto U, Renner U, Stalla G K. Front Neuroendocrinol. 1999;20:71–95. doi: 10.1006/frne.1998.0176. [DOI] [PubMed] [Google Scholar]
- 12.Ray D, Melmed S. Endocr Rev. 1997;18:206–228. doi: 10.1210/edrv.18.2.0297. [DOI] [PubMed] [Google Scholar]
- 13.Arzt E. J Clin Invest. 2001;108:1729–1733. doi: 10.1172/JCI14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCann S M, Kimura M, Yu W H, Mastronardi C A, Rettori V, Karanth S. Vitam Horm. 2001;63:29–62. doi: 10.1016/s0083-6729(01)63002-4. [DOI] [PubMed] [Google Scholar]
- 15.Hofland L J, de Herder W W, Waaijers M, Zuijderwijk J, Uitterlinden P, van Koetsveld P M, Lamberts S W. J Clin Endocrinol Metab. 1999;84:3336–3343. doi: 10.1210/jcem.84.9.6005. [DOI] [PubMed] [Google Scholar]
- 16.Massague J. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 17.Kawabata M, Imamura T, Miyazono K. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 18.Massague J. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 19.Chen C R, Kang Y, Massague J. Proc Natl Acad Sci USA. 2001;98:992–999. doi: 10.1073/pnas.98.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yagi K, Furuhashi M, Aoki H, Goto D, Kuwano H, Sugamura K, Miyazono K, Kato M. J Biol Chem. 2002;277:854–861. doi: 10.1074/jbc.M104170200. [DOI] [PubMed] [Google Scholar]
- 21.Bolognani F, Albariño C, Romanowski V, Carri N G, Goya R G. Eur J Endocrinol. 2001;145:497–503. doi: 10.1530/eje.0.1450497. [DOI] [PubMed] [Google Scholar]
- 22.Páez-Pereda M, Kovalovsky D, Hopfner U, Theodoropoulu M, Pagotto U, Uhl E, Losa M, Stalla J, Grubler Y, Missale C, et al. J Clin Invest. 2001;108:1123–1131. doi: 10.1172/JCI11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arzt E, Stelzer G, Renner U, Lange M, Muller O A, Stalla G K. J Clin Invest. 1992;90:1944–1951. doi: 10.1172/JCI116072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Páez-Pereda M, Missale C, Grubler Y, Arzt E, Schaaf L, Stalla G K. Mol Cell Endocrinol. 2000;167:99–106. doi: 10.1016/s0303-7207(00)00286-0. [DOI] [PubMed] [Google Scholar]
- 25.Hata A, Lagna G, Massague J, Hemmati-Brivanlou A. Genes Dev. 1998;12:186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monzen K, Shiojima I, Hiroi Y, Kudoh S, Oka T, Takimoto E, Hayashi D, Hosoda T, Habara-Onkubo A, Nakaoka T, et al. Mol Cell Biol. 1999;19:7096–7105. doi: 10.1128/mcb.19.10.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arzt E, Buric R, Stelzer G, Stalla J, Sauer J, Renner U, Stalla G K. Endocrinology. 1993;132:459–467. doi: 10.1210/endo.132.1.8419142. [DOI] [PubMed] [Google Scholar]
- 28.Newton C J, Arzt E, Stalla G K. Biochem Biophys Res Commun. 1994;205:1930–1937. doi: 10.1006/bbrc.1994.2896. [DOI] [PubMed] [Google Scholar]
- 29.Pereda M P, Goldberg V, Chervin A, Carrizo G, Molina A, Andrada J, Sauer J, Renner U, Stalla G K, Arzt E. Mol Cell Endocrinol. 1996;124:33–42. doi: 10.1016/s0303-7207(96)03924-x. [DOI] [PubMed] [Google Scholar]
- 30.Páez-Pereda M, Ledda M F, Goldberg V, Chervin A, Carrizo G, Molina H, Muller A, Renner U, Podhajcer O, Arzt E, Stalla G K. J Clin Endocrinol Metab. 2000;85:263–269. doi: 10.1210/jcem.85.1.6248. [DOI] [PubMed] [Google Scholar]
- 31.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone M V, Ametrano D, Zannini M S, Abbondanza C, Auricchio F. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith W C. Trends Genet. 1999;15:3–5. doi: 10.1016/s0168-9525(98)01641-2. [DOI] [PubMed] [Google Scholar]
- 33.Prall O W J, Rogan E M, Sutherland R L. J Steroid Biochem Mol Biol. 1998;65:169–174. doi: 10.1016/s0960-0760(98)00021-1. [DOI] [PubMed] [Google Scholar]
- 34.Delidow B C, Billis W M, Agarwal P, White B A. Mol Endocrinol. 1991;5:1716–1722. doi: 10.1210/mend-5-11-1716. [DOI] [PubMed] [Google Scholar]
- 35.Ramsdell J S. Endocrinology. 1991;128:1981–1990. doi: 10.1210/endo-128-4-1981. [DOI] [PubMed] [Google Scholar]
- 36.Coffey R J, Jr, Bascom C C, Sipes N J, Graves-Deal R, Weissman B E, Moses H L. Mol Cell Biol. 1988;8:3088–3093. doi: 10.1128/mcb.8.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massague J, Blain S W, Lo R S. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 38.Scully K M, Rosenfeld M G. Science. 2002;295:2231–2235. doi: 10.1126/science.1062736. [DOI] [PubMed] [Google Scholar]
- 39.Karsenty G. Genes Dev. 1999;13:3037–3051. doi: 10.1101/gad.13.23.3037. [DOI] [PubMed] [Google Scholar]
- 40.Massague J, Wotton D. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanagisawa J, Yanagi Y, Masuhiro Y, Suzawa M, Watanabe M, Kashiwagi K, Toriyabe T, Kawabata M, Miyazono K, Kato S. Science. 1999;283:1317–1321. doi: 10.1126/science.283.5406.1317. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson J A. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 43.Pettersson K, Gustafsson J A. Annu Rev Physiol. 2001;63:165–192. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- 44.Chen C R, Kang Y, Siegel P M, Massague J. Cell. 2002;110:19–32. doi: 10.1016/s0092-8674(02)00801-2. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda T, Yamamoto T, Muraguchi A, Saatcioglu A. J Biol Chem. 2001;276:42908–42914. doi: 10.1074/jbc.M105316200. [DOI] [PubMed] [Google Scholar]