Abstract
Aromatic amino acid hydroxylases (AAAH) typically use tetrahydrobiopterin (H4B) as the cofactor. The protozoan parasite Leishmania major requires biopterin for growth and expresses strong salvage and regeneration systems to maintain H4B levels. Here we explored the consequences of genetic manipulation of the sole L. major phenylalanine hydroxylase (PAH) to explore whether it could account for the Leishmania H4B requirement. L. major PAH resembles AAAHs of other organisms, bearing eukaryotic-type domain organization, and conservation of key catalytic residues including those implicated in pteridine binding. A pah− null mutant and an episomal complemented overexpressing derivative (pah−/+PAH) were readily obtained, and metabolic labeling studies established that PAH was required to hydroxylate Phe to Tyr. Neither WT nor overexpressing lines were able to hydroxylate radiolabeled tyrosine or tryptophan, nor to synthesize catecholamines. WT but not pah− parasites showed reactivity with an antibody to melanin when grown with L-3,4-dihydroxyphenylalanine (L-DOPA), although the reactive product is unlikely to be melanin sensu strictu. WT was auxotrophic for Phe, Trp and Tyr, suggesting that PAH activity was insufficient to meet normal Tyr requirements. However, pah− showed an increased sensitivity to Tyr deprivation, while the pah−/+PAH overexpressor showed increased survival and could be adapted to grow well without added Tyr. pah− showed no alterations in H4B-dependent differentiation, as established by in vitro metacyclogenesis, or survival in mouse or macrophage infections. Thus Leishmania PAH may mitigate but not alleviate Tyr auxotrophy, but plays no essential role in the steps of the parasite infectious cycle. These findings suggest PAH is unlikely to explain the Leishmania requirement for biopterin.
Keywords: trypanosomatid protozoa, tetrahydrobiopterin, aromatic amino acid hydroxylase, amino acid metabolism, virulence
1. Introduction
Leishmania is a genus of trypanosomatid protozoan parasites comprising more than 20 species responsible for a number of severe diseases of humans worldwide, infecting over 12 million people [1]. Leishmania are transmitted by the bite of Phlebotomine sand flies, and in the mammalian host reside primarily in an acidified phagolysosome of macrophages, where they must deflect host defenses and immune responses in order to survive [2]. As a deep-branching eukaryotic lineage, Leishmania exhibits considerable divergence in its metabolic enzyme repertoire [3], providing opportunities for basic studies as well as more practical efforts towards improved chemotherapy.
Here we focus on genetic studies of null and overexpression mutants of the sole Leishmania gene related to aromatic amino acid hydroxylases (AAAH). Our interest in AAAHs arose because their cofactor biopterin is an essential growth factor, distinct from folate, for several trypanosomatid species. In mammals tetrahydrobiopterin (H4B) is required by several enzymes of critical metabolic importance, including amino acid hydroxylases (AAAHs), nitric oxide synthase and the ether lipid cleavage monooxygenase [reviewed in 4, 5]. Leishmania and other trypanosomatids are general auxotrophs for pteridines [6, 7], and acquire biopterin from the host by salvage, first by uptake by the transporter BT1 [8, 9] and then reduction to H4B by the broad spectrum pteridine reductase PTR1 [10, reviewed by 11, 12]. The direct pteridine product of AAAH action is 4α-OH-H4B (carbinolamine) which is returned to H4B through the successive action of pteridine-4-carbinolamine dehydratase (PCD) and quininoid dihydropteridine reductase [QDPR; 4]. Trypanosomatids encode functional forms of both genes [13, 14].
Despite knowledge of H4B metabolism and requirements in Leishmania, the essential metabolic role of H4B is not established [15]. In other species, AAAH activity requires the amino acid substrate, molecular oxygen, and the reduced cofactor H4B [(6R)-L-erythro-5,6,7,8-tetrahydropterin; 16]. AAAHs catalyze the conversion of phenylalanine, tyrosine and/or tryptophan to tyrosine, 3,4-dihydroxyphenylalanine (L-DOPA), or 5-hydroxytryptophan respectively. Typically these activities are carried out by different enzymes, phenylalanine hydroxylase (PAH; EC 1.14.16.1), tyrosine hydroxylase (TyrH; EC 1.14.16.2) and tryptophan hydroxylase (TrpH; EC 1.14.16.4), although in some organisms these hydroxylases can show broader specificities [reviewed in 16, 17]. AAAH are widespread in evolution, being found in prokaryotes, protozoans, plants, fungi, and animals. In metazoans AAAH play critical roles in metabolism and neurotransmitter synthesis, and PAH participates in Phe catabolism in bacteria [18].
The identification of a putative H4B-dependent AAAH in the Leishmania genome raised the possibility that this activity might account for its H4B biopterin requirement. We report here studies that confirm the activity of Phe hydroxylase (PAH) in L. major, and characterize its role in parasite metabolism and infectivity.
2. Materials and methods
2.1. Chemicals and reagents
Dihydrobiopterin (H2B) was from Schircks Laboratories (Jona, Switzerland). Biopterin, epinephrine, norepinephrine, propanolol and RPMI 1640 vitamin solutions were from Sigma-Aldrich (St. Louis, USA). L-[5-H3] tryptophan, L-[3, 5-H3] tyrosine, and L-[2, 6-H3] phenylalanine) were obtained from Amersham Biosciences Corp. Phenol red solution and penicillin/streptomycin was from Invitrogen. Fetal calf serum was purchased from Bio-Whittaker and bovine serum albumin (Fraction V) from U. S. Biochemical Corp. M199 with Hanks s salts was from US Biologicals. Folate-deficient medium (fdM199) was custom manufactured by Invitrogen and is identical to M199 except lacking folate and thymidine [19].
2.2. Parasite and growth media
L. major Friedlin V1 (MHOM/JL/80/Friedlin) recovered from infected animals were used within 10 serial passages in vitro. Promastigotes were grown in M199 medium supplemented with 10 % heat-inactivated fetal bovine serum at 26°C [19]; for routine culture 2 μg ml−1 biopterin was added. Metacyclic promastigotes were purified from a stationary phase cells using the negative agglutination assay [20]. Amastigotes were harvested from BALB/c mice footpad lesion 3 weeks post infection.
In some experiments cells were grown in fdM199 (M199 medium lacking folate and thymidine supplemented with 0.66 % bovine serum albumin rather than serum). To deplete internal levels of biopterin, WT and KO cells were grown in fdM199 medium lacking biopterin for three passages (dilution of cells to ~ 2 × 105/ml and growth into stationary phase at about 2 × 107/ml). For differentiation, biopterin-depleted cells were incubated at a cell density of 2 × 105 cells/ml in fdM199 medium supplemented with biopterin (0.001 μg ml−1 or 2 μg ml−1), or without biopterin plus 100 μM epinephrine, 100 μM norepinephrine or 10 μM propanolol. Alternatively, an RPMI 1640 based medium was used, containing 1 % fetal bovine serum, 40 mM Hepes pH 7.4, 1X RPMI 1640 vitamin mix, 1X amino acid mixtures (lacking Phe, Tyr or Trp), 100 μM adenine, 1 μg ml−1biotin, 5 μg ml−1 hemin, 50 U ml−1 penicillin, 50 μg ml− 1streptomycin, 2 μg ml−1 biopterin, 5.37 mM KCl, 0.42 mM Ca(NO3)2•4H2O, 102.67 mM NaCl, 5.63 mM Na2HPO4, 0.41 mM MgSO4•7H2O, 23.81 mM NaHCO3, 11.11 mM glucose, and 0.0005% phenol red. Complete media additionally contained Phe (91 μM), Tyr (111 μM) and Trp (24 μM).
2.3. Southern and Northern blot analysis
L. major FV1 genomic DNA was isolated from the late logarithmic phase promastigotes by the LiCl method [22]. Genomic DNA was digested with the appropriate restriction enzymes and electrophoreses on 0.8 % agarose gels and transferred to nylon membranes. Total RNA of L. major promastigotes and amastigotes was isolated using the phenol/guanidine isothiocyanate reagent TRIzol™ (Invitrogen) according to the manufacturer’s instructions. Southern and Northern were performed following standard procedures and the hybridization probes were labeled with a [α-32P] dCTP by random-priming [23].
2.4. PAH gene cloning and sequencing
A PCR fragment spanning 870 nt to 953 nt of the PAH ORF) was obtained by PCR amplification (primers SMB1076 5′-GCCGGACATGGTTCACGACATCand SMB1077 5′-AGGCCGATCGTCTGGGTGAAG)and GSS clone lm94b04 [24] DNA template. This DNA was radiolabeled and used to screen an L. major Friedlin V1 cosmid library [25]. Three different cosmid clones containing PAH gene were obtained (c11p6, strain B4089; c10m17, strain B4086; c9o9, strain B4087). Following subcloning and mapping, the sequence of the PAH was determined using the ABI PRISM ™ BigDye Terminator Cycle Sequencing Ready Reaction kit (PE Applied Biosystems, Foster City, CA).
2.5. Mapping the 5′ Terminus of the mature PAH transcript
To amplify the 5′-end of PAH, L. major total RNA was used as a template for RT-PCR. Reverse transcription was performed with Superscript II reverse transcriptase (Invitrogen) following the manufacturer’s protocol. PCR was performed using Taq polymerase (Roche) with 30 cycles of denaturing at 94°C for 1 min, annealing at 50°C for 1 min and extension at 72°C for 2 min. Primers specific for the L. major spliced leader sequence (SMB936: 5′-ACCGCTATATAAGTATCAGTTCTGTACTTTA) and two specific primers, SMB1122 and 1117 located within the PAH coding region (SMB1122: 5′-TGCAGGGACGTGCGGCACCGCG and SMB1117: 5′-GTCGGGTACAGGCGGGTCAGGT). The PCR products were purified and sequenced directly.
2.6. Overexpression of PAH
The 1362-nt PAH open reading frame was amplified by PCR with Pfu polymerase (Stratagene) using primers SMB 1155 (5′-GCGGATCCACCATGCTGCTGCGCAGTCGTTTGT; the underline sequence corresponds to a BamHI site and the bold nucleotides correspond to a “Kozak” sequence), SMB 1156 (5′-CGGGATCCTTAGAAGGTGAAGTTTGTTCGC), and 50 ng of template genomic DNA. The amplified DNA fragment was digested and cloned into the BamHI site of the Leishmania expression vector pXG1a [26], yielding pXG-PAH (strain B4193), whose sequence was confirmed.
2.7. Molecular constructs for replacement of PAH alleles and transfection
The 5′-flanking region of the PAH gene was amplified by PCR using the primers SMB1589 (5′-CGGGGTACCGACGACAGACGTGCCG) and SMB1590 (5′-CCATCGATCTTATTCTCGAGGCAAGTCAGG) adding KpnI/ClaI sites, while the 3′ flanking region was PCR amplified using primers SMB1608 and 1592 adding BamHI/NotI sites. These fragments were digested with the appropriate enzymes and inserted successively into pBSKII (Promega, Madison, WI), yielding (pBS5′3′PAH). Then, BSD and HYG resistance ORFs were amplified individually with primers SMB1666 (5′-CCCAAGCTTATGGCCAAGCCTTTGTCTCAAGA)/SMB1667 (5′-CGGGATCCTTAGCCCTCCCACACATAACCAG) or SMB1665 (5′-CCCAAGCTTATGAAAAAGCCTGAACTCACCGC)/SMB1594 (5′-′CGGGATCCCTATTCCTTTGCCCTCGGACGA) respectively, which added HindIII and BamHI sites, and templates pXG-HYG [27] and pXG-BSD [28]. These fragments were digested appropriately and inserted into HindIII/BamHI digested pBS5′3′PAH, yielding the final replacement constructs pBS5′BSD3′PAH and pBS5′HYG3′PAH (strain B4696, strain B4692). DNAs were cut with NotI and KpnI, and the targeting fragment purified. WT parasites were electroporated with the PAH::ΔHYG targeting fragment derived from B4692 as described [21]. Transfectant colonies were obtained after plating on semisolid M199 medium containing hygromycin B (50 μg/ml), with typical frequencies. Three bearing heterozygous replacements (formally PAH/PAH::ΔHYG) were passaged through animals where they showed typical infectivity. One (clone 2) was selected and electroporated with the PAH::BSD targeting fragment, and, and transfectant colonies obtained after plating on semisolid M199 medium containing blasticidin (10 μg ml−1) and hygromycin B (50 μg ml−1), again at normal frequencies. Following molecular confirmation several lines (formally PAH::ΔHYG/PAH:: ΔBSD, referred to as pah− hereafter) where used to infect animals where they showed typical infectivity, as described elsewhere in the text. One of these (KO4) was selected and electroporated with pXG-PAH, and transfectant colonies obtained following plating on semisolid media containing G418 (10 μg ml−1), blasticidin (10 μg ml−1) and hygromycin B (50 μg ml−1). Several lines (formally, PAH:: ΔHYG/PAH:: ΔBSD [pXG-PAH], referred to as pah−/+PAH hereafter) were passaged through animals where they showed typical infectivity, and one (KO4-AB2) was selected.
2.8. Metabolic labeling
Leishmania major were grown in M199 medium until early log phase (2 × 106/ml), harvested by centrifugation, and washed twice and incubated further in modified RPMI 1640 medium whose aromatic amino acid contents was adjusted as follows. For Phe labeling, media contained 23 μM Phe and 8 μM Trp, while for Tyr and Trp labeling 23 μM Phe, 28 μM Tyr and 28 μM Trp were used. Cells were resuspended in 1 ml of medium at final concentration of 5 × 107/ml, and 10 μl of a 1 mCi/ml stock of radiolabeled L- (2,6-3H) Phenylalanine, L- (3,5-3H) Tyrosine or L- (5-3H) Trytophan added (Phe, 480 Ci/mmol, Tyr 55 Ci/mmol or Trp, 32 Ci/mmol). Cells were incubated for 2 hrs at 26°C, and then placed over 200 μl of dibutylphthalate and centrifuged 14,000 × g for 2 min. The upper aqueous layer was removed, and residual surface material was removed by washing twice with 1 ml of PBS. The oil layer was then removed, and the cells were recovered and resuspended in 100 μl of PBS.
For total amino acid uptake, 50 μl of suspended cells was lysed with 200 μl of 1% Triton X-100, added to 5 ml of Scintiverse II scintillation fluid, and used for liquid scintillation counting. For HPLC analysis, 50 μl of cell suspension was heated at 100° C for 10 min, and centrifuged at 14,000 × g for 15 min. Then, samples were derivatized as described [29] with some modifications. Briefly, 10–50 μl of cell lysate was mixed with 110 μl of borate buffer (0.2 M sodium borate, 1 mM EDTA, pH 8.8) and mixed by vortexing. Then 40 μl of AccQ-Fluor reagent (N-hydroxysuccinimidyl-6-aminoquinoyl carbamate Waters, USA) was then added to give a final volume of 200 μl, the sample was vortexed vigorously, and 50 μl of derivatized sample was used for chromatography
2.9. High performance liquid chromatography (HPLC)
A Waters HPLC and Millennium software was used for system control, data collection, and peak area calculation. HPLC columns and buffers were used as described [29], except Waters 600E gradient curve shape 7 was used instead of a linear gradient in order to improve separation of norepinephrine from valine. Unlabeled amino acids were included as standards as indicated. Fractions were collected at 1 min intervals, and 5 ml of Scintiverse II scintillation fluid was added prior to liquid scintillation counting.
2.10. Immunofluorescence
Ten μl suspensions containing 106 Leishmania cells grown with or without L-DOPA were dried on poly-L-lysine-coated slides. The slides were washed in PBS, incubated in Superblock® [Pierce, Rockford, IL] blocking buffer for 1 h at 37°C followed by incubation with 10 μg ml−1 of the melanin-binding monoclonal antibody 6D2 [30] for 1 h at 37°C. After washing, the slides were incubated with a 1:1000 dilution of tetramethyl rhodamine isothiocyanate (TRITC) -conjugated goat anti-mouse IgM (Southern Biotechnologies Associates, Inc; Birmingham, AL) for 1 h at 37° C. Slides were washed, mounted using a 50% glycerol, 50% PBS, and 0.1 M N-propyl gallate solution, and viewed with an Olympus AX70 microscope (Melville, NY) equipped with a TRITC filter. Negative controls were incubated with mAb 5C11, which binds mycobacterial lipoarabinomannan [31] as the primary Ab, or TRITC-labeled Ab alone.
2.11. Melanin isolation
Promastigotes were suspended in 1.0 M sorbitol-0.1 M sodium citrate, pH 5.5. Cell wall lysing enzymes (from Trichoderma harzianum; Sigma-Aldrich, St. Louis) were added (10 mg ml−1) and suspensions were incubated at 30°C overnight. Cells were collected by centrifugation, washed with PBS, and incubated with 4 M guanidine thiocyanate 12 h. The particulate was treated with 1 mg ml−1 proteinase K (10 mM Tris, 1.0 mM CaCl2, and 0.5% SDS, pH 7.8) at 37°C overnight and then extracted three times with chloroform. The debris was collected, washed with PBS, and boiled in 6.0 M HCl for 1 h.
2.12. Mouse infectivity tests
BALB/c mice were from Charles River Laboratories Inc. (Wilmington, MA) and CBA/J mice from the Jackson Laboratory (Bar Harbor, Me). All studies were performed using protocols approved by the institutional animal studies committee. Parasites were grown to stationary phase in M199 medium, and groups of BALB/c mice were injected subcutaneously (s.c.) into the footpads with 106, 105, 104 parasites per mice [32]. Lesions were monitored by measuring the thickness of the footpad with a Vernier caliper. Lesion parasites were enumerated in the infected tissue by a limiting dilution assay [33].
2.13. Macrophage Infections
Infection of peritoneal macrophages with C3-opsonized parasites with performed as described [34]. The medium was changed daily, and at days 1, 2, 3 postinfection, intracellular parasites were visualized in formaldehyde-fixed macrophages by nuclear staining with Hoechst 33342 (0.5 μg ml−1).
3. Results
3.1 Identification and properties of an L. major PAH gene
We identified a candidate phenylalanine hydroxylase gene in blast searches of data obtained from a whole genome shotgun library [24]. A radiolabeled probe was used to screen an L. major cosmid library, and the relevant regions of several cosmids were sequenced. The gene encoded a predicted protein of 453 aa/50.4 kDa (Fig. 1) and was given the gene symbol PAH, as it was subsequently shown below to be required for PAH activity. Southern blot analysis showed it to be present in a single copy (Fig. S1), in good agreement with the current L. major genome assembly, where PAH bears the systematic gene identifier LmjF28.1280 [35].
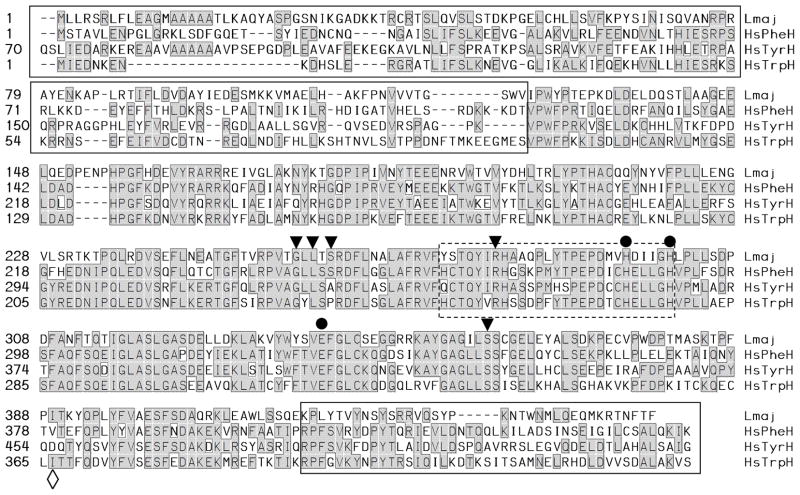
Fig. 1. Alignment of L. major PAH with human phenylalanine, tyrosine and tryptophan hydroxylases.
The predicted amino acid sequence of L. major PAH (Lmaj) was aligned with human phenylalanine, tryptophan and tyrosine hydroxylase (HsPheH, TyrH and TrpH; accession numbers are provided in the legend to Fig. S3). Residues identical in two or more sequences are highlighted in gray boxes. Residues implicated in iron binding are marked by a ●, residues implicated in substrate or cofactor binding by a ▼, and residues associated with amino acid specificity by a ◇. The presumptive N terminal ‘regulatory’ and C-terminal ‘oligomerization’ domains are marked by a solid box, while the region homologous to the pteridine binding region of other AAAH is marked by a dashed box.
L. major PAH displayed an overall 42–51 % identity to AAAH from mammals, Drosophila or C. elegans. Mammalian AAAHs contain a variable N terminal domain comprising about 1/3 of the protein that mediates regulatory functions, a central conserved catalytic domain, and a short C-terminal domain mediating oligomerization [reviewed in 16, 17]. Similarly, LmjPAH showed a divergent N terminal domain comprising amino acids 1–123, a more conserved catalytic domain comprising amino acids 124–436, followed by a divergent C-terminal domain (Fig. 1). Recently two PAHs were described in Toxoplasma gondii, whose predicted proteins bearing signal sequences potentially indicative of secretion [36]. However, L. major PAH lacks an N-terminal extension (Fig. 1) and several computational methods did not predict a signal sequence for the Leishmania PAH.
Structural motifs characteristic of aromatic amino acid hydroxylases were found in the central catalytic region of LmjPAH, including the iron binding site (His295, His301, and Glu331 in LmjPAH) [37], and conserved substrate and cofactor binding sites (residues 274–301) [38]. LmjPAH contains Asp at the position corresponding to Glu286 of rat PheH, which has been implicated in H4B binding [39]. While amino acid hydroxylases often show cross−activity to varying extents, and several studies have shown it is difficult to reliably predict amino acid specificity from amino acid sequences alone, some trends have been observed [16]. Several amino acid residues determined the substrate specific activity for tyrosine or tryptophan hydroxylase were not found in LmPAH. These include the position corresponding to Y235 and F313 in tryptophan hydroxylase [40] and D425 of tyrosine hydroxylase [41].
3.2. Structure and expression of PAH mRNA during the infectious cycle
In Leishmania and related trypanosomatid protozoans, every mRNA contains a 39-nt ‘spliced-leader’ at its 5′ end added by trans-splicing [42]. We used RT-PCR with a spliced-leader and two primers specific for PAH to map the 5′ trans-splice acceptor site of the PAH mRNA to an AG dinucleotide 141 nt 5′ of the predicted AUG initiation codon, which was also the first AUG (data not shown). Northern blots revealed a single 4 kb transcript in all stages of the infectious cycle (early log promastigote, late log promastigote, metacyclic promastigote and amastigote) with little variation during development (Fig. 2). A similar result was obtained with the mRNA encoding the H4B regenerating enzyme QDPR [13].
Fig. 2. Expression of PAH mRNA during the Leishmania major infectious cycle.
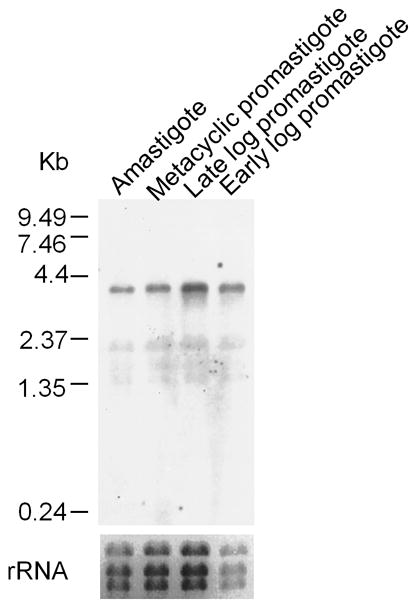
Northern blot analysis of total RNAs probed with the LmPAH coding region sequence are shown. The autoradiogram is shown in the upper panel with the single ~ 4-kb LmPAH transcript. Molecular size markers (kb) are shown to the left. The lower panel shows the ethidium bromide-stained gel showing the three rRNAs as a loading control.
3.3. Generation of PAH null mutants by targeted gene deletion
We were unable to express in an enzymatically active form in E. coli, or in an active form in vitro, despite many attempts. These included varying expression conditions or host cells, tests of alternative pteridines including folates, or tests of truncations removing the poorly conserved N terminal ‘regulatory’ domain, or this as well as the C-terminal domain; several such variations had proven successful in studies of other species’ AAAH. Thus we turned to studies of PAH null mutants, or parasites over-expressing PAH, to explore its potential roles and activities in vivo.
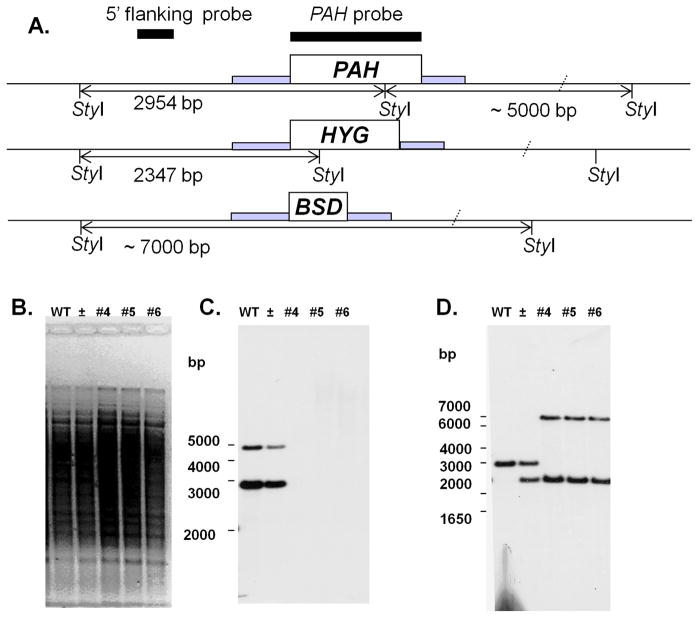
As Leishmania species are predominantly diploid, two successive rounds of gene targeting were required to generate null mutants [27]. The first PAH allele was replaced by the hygromycin B resistance marker (HYG) and the second was replaced with the blasticidin (BSD) resistance marker (Fig. 3A). Southern blot analysis with a PAH probe confirmed that the PAH gene had been completely deleted (Fig. 3C), while hybridization with a flanking probe confirmed the replacements had occurred as planned (Fig. 3D). Notably, homozygous replacement (pah−) lines were obtained a typical frequencies, and grew normally in standard M199 culture media thereafter. Several independent lines were analyzed, all of which had similar growth properties (not shown). We restored PAH expression by transfection of an episomal multicopy vector, yielding lines termed pah−/+PAH, which additionally exhibit PAH overexpression.
Fig. 3. Generation of L. major PAH null mutants.
(A) Restriction map of the native PAH locus and planned HYG and BSD replacements. The position of StyI sites and predicted fragment sizes are shown. Hybridization probes are shown by black bars, codon regions by open boxes, and regions contained within the targeting fragment by gray bars. (B–D) Southern blot analysis of wild type (WT), heterozygous (±) and PAH null mutant lines (#4, 5, and 6). DNA was subjected to Southern blot analysis using the radiolabeled PAH ORF (panel C) or a 5′ flanking (panel D) probes shown in panel A. Panel B shows the ethidium bromide stained gel prior to transfer.
3.4. In vivo detection of PAH-dependent phenylalanine hydroxylase activity by metabolic labeling
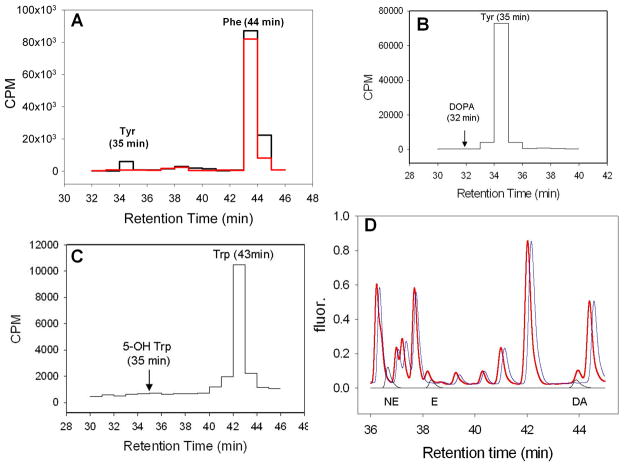
Leishmania were grown to logarithmic phase, collected, washed twice, and inoculated into media containing radiolabeled phenylalanine, tyrosine or tryptophan. After 2 hr, samples were collected, deproteinized, and separated by HPLC after addition of the appropriate standards (Fig. 4A–C). When labeled with [3H]-Phe, WT but not pah− extracts showed incorporation into a peak comigrating with the free Tyr standard (Fig 4A). In contrast, no incorporation above background was seen using [3H]-Tyr or [3H]-Trp, into their predicted products DOPA or 5-hydroxy-tryptophan (Fig. 4B, C). Since limiting concentrations of radiolabeled essential amino acids were used in order to maximize the specific activity, and free amino acids are used in protein synthesis, in preliminary experiments we varied the time of labeling from 1 to 4 h. As before no further metabolism of radiolabeled Tyr or Trp was detected, while [3H]-Tyr formation from [3H]-Phe was maximal at 2 hr. Other than Tyr formation, no differential was seen between WT and pah−, or between the pah−/+PAH overexpressor (data not shown). These data indicate that the Leishmania PAH is required for phenylalanine but not tyrosine or tryptophan hydroxylase activity. Potentially, PAH possess a low level of tyrosine hydroxylase activity not evident in our studies above. Thus we sought but were unable to detect by HPLC potential downstream catecholamine metabolites (norepinephrine, epinephrine, dopamine) in WT extracts (Fig 4D).
Fig. 4. Metabolic labeling with aromatic amino acids and catecholamine analysis of L. major.
Panels A–C. WT (black lines) or pah− (red line) were grown in the presence of [3H] amino acids, and incorporation into free Phe, Tyr or Trp in deproteinized cell lysates was determined by liquid scintillation counting following derivatization and HPLC separation. Panel D. Analysis of L. major for the presence of catecholamines. Deproteinized lysates were separated by HPLC following derivatization as described in the Methods. The fluorescence traces are shown for WT (blue) and WT mixed with norepinephrine (NE), ephinephrine (E) and dopamine (DA) standards (red). Offset below these are traces (black) for separation of NE, E, and DA run individually.
Total uptake and incorporation of radiolabeled Phe after 2 hr into free Phe and Tyr were quantified in the WT, pah− and pah−/+PAH lines (Table 1). Total Phe uptake/incorporation was somewhat less in pah− (75% WT) and elevated in pah−/+PAH (129% WT), although these differences were not significant. Free Phe was somewhat reduced in pah− (78% WT) and more strongly reduced in the pah−/+PAH line (27% WT); again the differences were not significant. As shown in Fig 4A, pah− showed no incorporation of Phe into free Tyr which was strongly significant (P<0.01), while pah−/+PAH showed a 2.4-fold increase.
Table 1.
Metabolic labeling studies of L. major with [3H] Phe.
| Cell Line | |||
|---|---|---|---|
| WT | pah− | pah−/+PAH | |
| Total Phe Uptake/incorporation | 2433 ± 251 (n = 3) | 1820 ± 276 ** (n = 5) | 3140 ± 190 * (n = 4) |
| Free Phenylalanine | 129 ± 49 | 101 ± 11 | 34.5 ± 14.4 * |
| Free Tyrosine | 6.5 ± 1.7 | 0 (Not detectable) *** | 15.3 ± 10.7 |
The values shown are pmol [3H]-Phe or Tyr/2.5×107 cells). Statistical significance from WT is indicated as
(P < 0.1),
(P < 0.05) or
(P < 0.01).
3.5. PAH and aromatic amino acid growth requirements
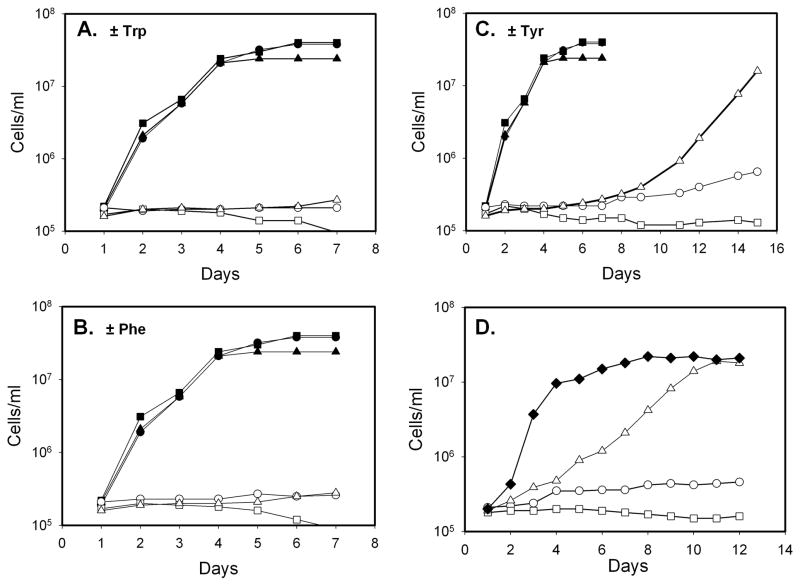
To probe the consequences of PAH depletion or overexpression, parasites were inoculated into semidefined medium, or this lacking phenylalanine, tryptophan or tyrosine. The WT and pah− lines were incapable of sustained proliferation in media lacking any of the aromatic acids (Fig. 5A–C), consistent with the view that Leishmania are auxotrophic for aromatic amino acids [43]. Unlike WT cells which arrested at constant cell numbers, pah− showed a tendency towards decreased cell numbers over time (Fig. 5A–C).
Fig. 5. Growth of L. major WT, pah− and pah−/+PAH lines in aromatic amino acid-deficient media.
The growth of parasites was measured following inoculation at 2 × 105/ml on day 1; all studies were performed at least three times, with a representative experiment shown. Panel A, growth in media containing (dark symbols) or lacking Trp (light symbols). Panel B, growth in media containing (dark symbols) or lacking (open symbols) Phe. Panel C., growh in media containing (dark symbols) or lacking (open symbols) Tyr. Panel D, growth in media lacking Tyr. Cell lines were WT (●, ○), pah− (■, □) or pah−/+PAH (▲, △), or pah−/+PAH adapted by six serial passages of growth in media lacking Tyr (◆).
After a substantial delay of ~8 days, the pah−/+PAH overexpressor was able to resume growth in Tyr-deficient media, although more slowly than in the presence of Tyr (Fig 5C). These parasites were adapted further by passage in Tyr-deficient media (six passages of 1:100 serial dilutions), and then retested. These P6 pah−/+PAH parasites now exhibited growth comparable to WT in the presence of Tyr (Fig. 5D). Adaptation was dependent on the presence of the episomal PAH gene, as WT parasites never increased in their rate of growth (not shown). The basis of adaptation was not studied further but its dependency on episomal PAH suggests that increased plasmid copy number and PAH expression may account for this finding, seen originally in ‘super-selections’ of L. major episomal vectors [21]. These data provide independent confirmation of the PAH activity of PAH, through its role in provision of Tyr under limiting conditions, and alleviation of this requirement when overexpressed.
3.6. Infectivity of pah− L. major in mouse or macrophage infections
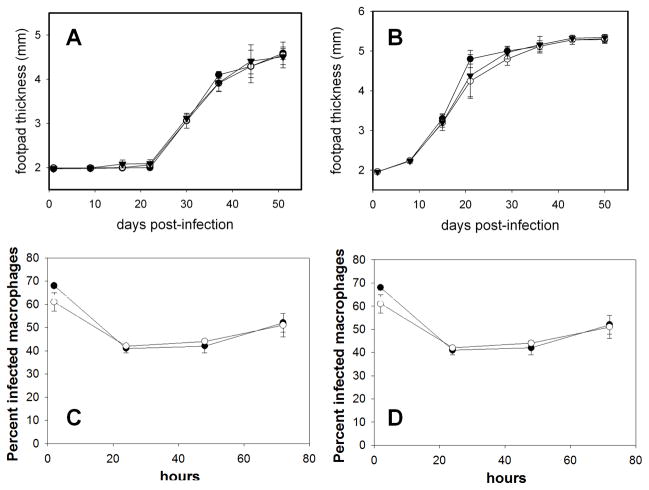
The ability of pah− mutant parasites to cause lesions in susceptible BALB/c mice was investigated by inoculating infectious stationary phase promastigotes (104 – 106) into footpads, and monitoring parasite-induced pathology by measuring footpad thickness over time. For the WT, pah− and pah−/+PAH lines, there was no difference in the time until lesion pathology appeared, nor in the progression of lesion pathology thereafter (Fig. 6A–B). Similarly, when inoculated into genetically resistant CBA/J mice, which show transient lesion pathology, all three lines behaved identically (data not shown).
Figure 6. Virulence of pah− promastigotes is not attenuated mouse or macrophage infections.
Panels A & B. 104 (A) or 106 (B) stationary-phase WT or pah− parasites were inoculated individually into the footpad of BALB/c mice, and footpad thickness was measured over time. The average and standard deviation from three mice for a representative experiment is shown. Panels C & D, macrophage infections. Survival of complement-opsonized stationary-phase WT or pah− is shown. Panel A, B: WT (●), and pah− lines KO4 (○) or KO5 (▼). Panel C, D: WT (●) or pah− KO4 (○). The average and standard deviation of triplicate determinations from a representative experiment is shown.
The ability of WT and pah− Leishmania to survive within peritoneal macrophages was assessed following infection of complement-opsonized stationary phase parasites. Parasitemia was monitored by the percent infected macrophages (Fig. 6C) and the number of parasites per 100 macrophages (Fig. 6D). Again, no difference in parasite survival was seen.
3.7. Metacyclogenesis
Previously we showed that H4B levels were associated with increased differentiation into the infective metacyclic form, which occurs normally following entry into stationary phase [44]. Since PAH activity could affect H4B levels, we explored the effect of PAH depletion or overexpression on metacyclogenesis. Tests were performed in folate-depleted M199 media (fdM199) containing either minimal (0.001 μg/ml) or saturating (2 μg/ml) concentrations of biopterin. As before, no differences in growth rate were observed, and metacyclogenesis was elevated under low-biopterin conditions (10–11% vs 4.5 – 5%; Fig. S2, or data not shown). However, no differences were seen in metacyclogenesis between WT or pah− under either condition (Fig. S2). Similar tests showed no effect of epinephrine (100 μM), norepinephrine (100 μM) or propanolol (10 μM) on either parasite growth or differentiation, for either WT or pah− (Fig. S2). We conclude that neither PAH or the presence of potential downstream effectors arising from PAH activity have any effect on metacyclogenesis, even when grown in the minimum level of biopterin required to support normal growth.
3.8. Reactivity with anti-Melanin antibody MAb 6D
Melanin is a downstream metabolite of AAAH activity in many species [45, 46] and we used reactivity with the melanin antibody MAb 6D2 to reveal its presence in Leishmania. While WT parasites showed no reactivity, comparable to isotypic controls (Fig. 7A or data not shown), when grown in media containing 250 μM DOPA, strong reactivity was observed (Fig. 7C). In contrast, pah− parasites showed no reactivity when grown in the presence of DOPA (Fig. 7B). Restoration of PAH expression in the pah−/+PAH line restored MAb 6D2 reactivity (Fig. 7D). We observed in some but not all experiments that the cells were pigmented in PAH-expressing cells grown in the presence DOPA. We sought to confirm that the reactive material was indeed melanin, using a protocol which solubilizes most cellular components but leaves insoluble melanin intact, but were unsuccessful with WT cells grown in the presence of DOPA (not shown).
Figure 7. Immunofluorescence analysis using anti-melanin antibody MAb 6D2.
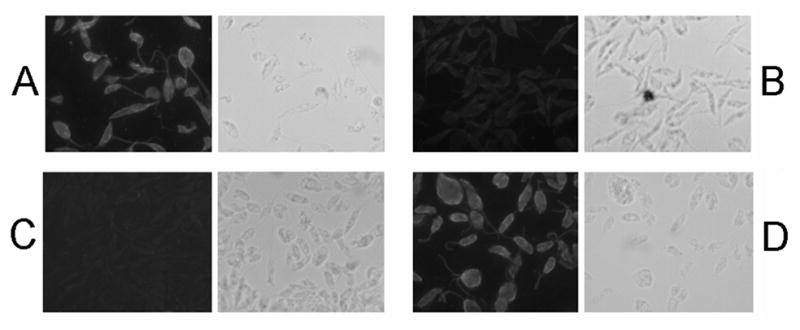
Panel A, WT grown in the presence of L-DOPA; Panel B, pah− grown in the presence of L-DOPA; Panel C, WT grown in the absence of L-DOPA; and Panel D, pah−/+PAH grown in the presence of L-DOPA. When added the L-DOPA concentration was 250 μM.
4. Discussion
In this work we identified and explored potential roles for L. major PAH. As we were unable to express active PAH for biochemical characterization, we relied primarily on the predicted properties based on the PAH protein sequence, and the characterization of both a pah− null mutant and a PAH overexpressor obtained following transfection of a multicopy episomal PAH expression vector. Collectively the aromatic amino acid hydroxylase family shares many physical, structural and catalytic properties [4, 5, 16]. The predicted L. major PAH protein showed a typical mammalian-type AAAH domain structure, with a highly conserved central catalytic core flanked by less conserved N terminal and C terminal regions, implicated in regulatory and oligomerization roles in other AAAHs (Fig. 1). The L. major PAH central catalytic core showed good conservation of catalytic residues and motifs seen in other AAAHs. Importantly, these residues include those responsible for binding H4-biopterin.
Several other protozoans bear AAAH genes, including the apicomplexan parasite Toxoplasma which has two genes encoding potentially secreted Phe and Trp hydroxylase activity [36], and the cellular slime mold Dictyostelium [47]. Phenylalanine hydroxylases also occur sporadically throughout prokaryotes [4, 18]. Evolutionarily, L. major PAH falls as expected near the base of the eukaryotic radiation (Fig. S3). While all Leishmania species genomes reported to date encode a PAH ortholog, it is absent from the genomes of both African and South American trypanosomes. Since a PAH gene occurs in the free-living Kinetoplastid relative Bodo saltans (family Bodonidae; Fig. S3), we infer that PAH was lost in the common ancestor of trypanosomes following their divergence from Leishmania. However, the genome of African trypanosomes predicts a PCD gene and trypanosomes express high levels of QDPR activity [13]. This would be consistent with the idea that H4B is required for essential processes in Leishmania and trypanosomes, other than as a cofactor for AAAH activity.
Characterization of the L. major pah− null mutant showed phenotypes firmly establishing the requirement for PAH for phenylalanine hydroxylase activity. While WT parasites converted radiolabeled Phe to Tyr, the pah− mutant was unable to do so (Fig. 4A, Table 1), and correspondingly, the pah−/+PAH overexpressor showed an elevated ability to do so (Table 1). Despite this activity, WT L. major were auxotrophic for Tyr as well as Phe and Trp (Fig. 5A–C), suggesting that WT PAH levels were insufficient for metabolic needs. Notably, WT and PAH overexpressing parasites showed reproducibly higher susceptibility to Phe or Trp deprivation than for Tyr deprivation (Fig. 5–C). Moreover, the pah−/+PAH episomal overexpressor showed only partial growth inhibition, and could be readily adapted for normal growth without Tyr (Fig. 5C, D). These data suggest that WT PAH activity can only partially alleviate Tyr insufficiency, and is able to facilitate cell survival but not full growth. The ability of PAH overexpression to rescue Tyr auxotrophy of the pah− mutant firmly establishes the PAH activity of LmjPAH.
In contrast to the PAH-dependent Phe hydroxylase activity, Tyr or Trp hydroxylase activity was not detected by in vivo radiolabeling, using either WT, pah− or the pah−/+PAH overexpressor. This is consistent with LmjPAH amino acid sequence, which shows residues more typical of AAAHs with Phe rather than Tyr hydroxylase activity (Fig. 1). Correspondingly, PAH expression levels did not alter growth under Phe or Trp deprivation (Fig. 5A, B). These data thus suggest that Leishmania PAH is specific for Phe.
4.1. A melanin-like substance in Leishmania?
Curiously, we were able to show reactivity of Leishmania against an antibody to melanin, which was dependent on the presence of both PAH and exogenous DOPA (Fig. 7), and in some experiments the Leishmania appeared pigmented. However, we failed to recover insoluble acid-resistant pigment characteristic of melanin [30], suggesting that the reactive substance was unlikely to be authentic melanin. A number of melanin-like substances have been described, arising from a variety of diphenolic or other precursors [48]. In the fungus Cryptococcus neoformans, melanin plays important roles in virulence [45]. However, since Leishmania requires PAH to make the melanin-like substance, yet does not require PAH for differentiation or infectivity in mice or macrophages (Fig. 7), a role for the melanin-like substance in Leishmania virulence seems unlikely.
4.2. PAH null mutants remain viable and virulent
Given the importance of H4B to Leishmania viability and virulence, and that melanin-like substances have been implicated in host-pathogen interactions in several species [36, 46, 49], we asked whether PAH-dependent metabolites played a role in the Leishmania infectious cycle. First we examined the ability of the parasite to differentiate to infectious metacyclic parasites in vitro, a process known to be up-regulated by low H4B levels in L. major [44]. We confirmed this result, virulent L. major FV1 line studied here, but the percent metacyclics was identical between WT and pah− lines when grown in either high or minimal biopterin levels, and nor was it altered in the presence of downstream metabolites including catecholamines or inhibitors (Figure S2). Similarly, the pah− line behaved identically to WT following infections of primary macrophages in vitro, or in infections of susceptible and resistant mice in vivo (Fig. 6 or data not shown). Finally, in preliminary studies the pah− line survived well within sand flies, the insect vector of leishmaniasis (D. Sacks, L-FL and SMB, unpublished data).
Thus, while under some circumstances PAH can partially mitigate the Tyr growth requirement of L. major, it is not essential for normal growth nor virulence across the Leishmania infective cycle. Similarly, the PAH gene is absent in trypanosomes, which like Leishmania require H4B [50]. Future studies may consider other metabolic roles for PAH, perhaps in Phe anabolism, drug resistance or in other metabolic pathways such as thioether metabolism [45, 51]. However, since the pah− mutant grows normally in vitro and in vivo, it seems unlikely that PAH activity or PAH-dependent metabolites can account completely for the strong biopterin growth requirement of Leishmania.
Supplementary Material
Genomic DNA isolated from L. major FV1 was digested with the indicated panel of restriction endonuclease, and subjected to Southern blot analysis using a radiolabeled LmjPAH coding sequence. The sizes of molecular weight standards (kb) are shown.
Leishmania WT and KO cells were grown individually under ‘standard/high’ or ‘minimal/low’ concentrations of H4B (H, 2, and L, 0.001 μg/ml), epinephrine (EP; 100 μM), norepinephrine (NP; 100 μM) or propanolol (PPL, 10 μM) as described in the text, and the percent of metacyclics was measured after 3 days in stationary phase. The average and standard deviation of duplicate samples from a representative experiment is shown.
Evolutionary relationships amongst the indicated AAAH were calculated using the ClustalW algorithm [65] included in the Lasergene package (DNAStar, Inc.). Where known, the aromatic amino acid specificity is indicated by the one-letter amino acid code. D. melanogaster PheH/TrpH (P17276) and TyrH (NP_476897); C. elegans PheH (P90925), TrpH (NP_495584), and TyrH (NP_871903); Human TyrH (P07101), TrpH (P17752) and TyrH (P00439); Leishmania major PAH (AY273788); L. infantum (XP_001470169); L. braziliensis (XP_001566169); Bodo saltans (ACI15907); Toxoplasma gondii 2 (ACB99414); Dictyostelium discoideum (XP_641959); Pseudomonas (NP_249563); Chromobacterium (NP_902850); and Caulobacter (NP_420423).
Acknowledgments
We thank A. H. Fairlamb (U. Dundee) and A. Hanson (U. Florida Gainesville) for discussions, S. C. Daubner and P.F. Fitzpatrick (Texas A&M University) and A. Pribat (U. Florida Gainesville) for assistance and advice in attempts to express active LmjPAH, S. Hickerson for instruction in mouse infectivity tests and macrophage infections, D.L. Sacks (NIH) for preliminary studies of sand fly infectivity, and Tim J. Vickers for comments on this manuscript. This work was supported by National Institutes of Health Grants AI21903 (L-FL, SOK, SMB) and AI52733 (AC, JDN).
Abbreviations
- PAH
phenylalanine hydroxylase
- AAAH
aromatic amino acid hydroxylase
- LmjPAH/LmPAH
Leishmania major phenylalanine hydroxylase gene/enzyme
- H2B
dihydrobiopterin
- H4B
tetrahydrobiopterin
- bp
base pair(s)
- ORF
open reading frame
- nt
nucleotide(s)
- PCR
polymerase chain reaction
- Phe
phenylalanine
- Tyr
tyrosine
- Trp
tryptophan
- WT
wild-type
- L-DOPA
L-dihydroxyphenylalanine
Footnotes
The nucleotide sequences reported in this paper have been submitted to the GenBank™/EBI Data bank with accession number AY273788
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Desjeux P. Leishmaniasis: current situation and new perspectives. Comparative immunology, microbiology and infectious diseases. 2004;27:305–18. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nature Immunol. 2002;2:845–58. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 3.Opperdoes FR, Coombs GH. Metabolism of Leishmania: proven and predicted. Trends Parasitol. 2007;23:149–58. doi: 10.1016/j.pt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347(Pt 1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 5.Werner-Felmayer G, Golderer G, Werner ER. Tetrahydrobiopterin biosynthesis, utilization and pharmacological effects. Curr Drug Metab. 2002;3:159–73. doi: 10.2174/1389200024605073. [DOI] [PubMed] [Google Scholar]
- 6.Petrillo-Peixoto M, Beverley SM. In vitro activity of sulfonamides and sulfones against Leishmania major promastigotes. Antimicrob Agents Chemother. 1987;31:1575–8. doi: 10.1128/aac.31.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck JT, Ullman B. Nutritional requirements of wild-type and folate transport-deficient Leishmania donovani for pterins and folates. Mol Biochem Parasitol. 1990;43:221–30. doi: 10.1016/0166-6851(90)90147-e. [DOI] [PubMed] [Google Scholar]
- 8.Kundig C, Haimeur A, Legare D, Papadopoulou B, Ouellette M. Increased transport of pteridines compensates for mutations in the high affinity folate transporter and contributes to methotrexate resistance in the protozoan parasite Leishmania tarentolae. EMBO J. 1999;18:2342–51. doi: 10.1093/emboj/18.9.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham ML, Beverley SM. Pteridine salvage throughout the Leishmania infectious cycle: implications for antifolate chemotherapy. Mol Biochem Parasitol. 2001;113:199–213. doi: 10.1016/s0166-6851(01)00213-4. [DOI] [PubMed] [Google Scholar]
- 10.Bello AR, Nare B, Freedman D, Hardy L, Beverley SM. PTR1: a reductase mediating salvage of oxidized pteridines and methotrexate resistance in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A. 1994;91:11442–6. doi: 10.1073/pnas.91.24.11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouellette M, Drummelsmith J, El-Fadili A, Kundig C, Richard D, Roy G. Pterin transport and metabolism in Leishmania and related trypanosomatid parasites. Int J Parasitol. 2002;32:385–98. doi: 10.1016/s0020-7519(01)00346-0. [DOI] [PubMed] [Google Scholar]
- 12.Nare B, Luba J, Hardy LW, Beverley S. New approaches to Leishmania chemotherapy: pteridine reductase 1 (PTR1) as a target and modulator of antifolate sensitivity. Parasitology. 1997;114 (Suppl):S101–10. [PubMed] [Google Scholar]
- 13.Lye LF, Cunningham ML, Beverley SM. Characterization of quinonoid-dihydropteridine reductase (QDPR) from the lower eukaryote Leishmania major. J Biol Chem. 2002;277:38245–53. doi: 10.1074/jbc.M206543200. [DOI] [PubMed] [Google Scholar]
- 14.Naponelli V, Noiriel A, Ziemak MJ, Beverley SM, Lye LF, Plume AM, et al. Phylogenomic and functional analysis of pterin-4a-carbinolamine dehydratase family (COG2154) proteins in plants and microorganisms. Plant Physiol. 2008;146:1515–27. doi: 10.1104/pp.107.114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nare B, Garraway LA, Vickers TJ, Beverley SM. PTR1-dependent synthesis of tetrahydrobiopterin contributes to oxidant susceptibility in the trypanosomatid protozoan parasite Leishmania major. Curr Genet. 2009;55:287–99. doi: 10.1007/s00294-009-0244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Annu Rev Biochem. 1999;68:355–81. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- 17.Hufton SE, Jennings IG, Cotton RG. Structure and function of the aromatic amino acid hydroxylases. Biochem J. 1995;311 (Pt 2):353–66. doi: 10.1042/bj3110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao G, Xia T, Song J, Jensen RA. Pseudomonas aeruginosa possesses homologues of mammalian phenylalanine hydroxylase and 4 alpha-carbinolamine dehydratase/DCoH as part of a three-component gene cluster. Proc Natl Acad Sci U S A. 1994;91:1366–70. doi: 10.1073/pnas.91.4.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nare B, Hardy LW, Beverley SM. The roles of pteridine reductase 1 and dihydrofolate reductase-thymidylate synthase in pteridine metabolism in the protozoan parasite Leishmania major. J Biol Chem. 1997;272:13883–91. doi: 10.1074/jbc.272.21.13883. [DOI] [PubMed] [Google Scholar]
- 20.da Silva R, Sacks DL. Metacyclogenesis is a major determinant of Leishmania promastigote virulence and attenuation. Infect Immun. 1987;55:2802–6. doi: 10.1128/iai.55.11.2802-2806.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapler GM, Coburn CM, Beverley SM. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990;10:1084–94. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medina-Acosta E, Cross GA. Rapid isolation of DNA from trypanosomatid protozoa using a simple ‘mini-prep’ procedure. Mol Biochem Parasitol. 1993;59:327–9. doi: 10.1016/0166-6851(93)90231-l. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press; 1989. Molecular cloning: a laboratory manual; pp. 1–1000. [Google Scholar]
- 24.Akopyants NS, Clifton SW, Martin J, Pape D, Wylie T, Li L, et al. A survey of the Leishmania major Friedlin strain V1 genome by shotgun sequencing: a resource for DNA microarrays and expression profiling. Mol Biochem Parasitol. 2001;113:337–40. doi: 10.1016/s0166-6851(01)00227-4. [DOI] [PubMed] [Google Scholar]
- 25.Ryan KA, Dasgupta S, Beverley SM. Shuttle cosmid vectors for the trypanosomatid parasite Leishmania. Gene. 1993;131:145–50. doi: 10.1016/0378-1119(93)90684-u. [DOI] [PubMed] [Google Scholar]
- 26.Ha DS, Schwarz JK, Turco SJ, Beverley SM. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol. 1996;77:57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 27.Cruz A, Coburn CM, Beverley SM. Double targeted gene replacement for creating null mutants. Proc Natl Acad Sci U S A. 1991;88:7170–4. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goyard S, Beverley SM. Blasticidin resistance: a new independent marker for stable transfection of Leishmania. Mol Biochem Parasitol. 2000;108:249–52. doi: 10.1016/s0166-6851(00)00210-3. [DOI] [PubMed] [Google Scholar]
- 29.Coppi A, Merali S, Eichinger D. The enteric parasite Entamoeba uses an autocrine catecholamine system during differentiation into the infectious cyst stage. J Biol Chem. 2002;277:8083–90. doi: 10.1074/jbc.M111895200. [DOI] [PubMed] [Google Scholar]
- 30.Rosas AL, Nosanchuk JD, Feldmesser M, Cox GM, McDade HC, Casadevall A. Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect Immun. 2000;68:2845–53. doi: 10.1128/iai.68.5.2845-2853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glatman-Freedman A, Martin JM, Riska PF, Bloom BR, Casadevall A. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J Clin Microbiol. 1996;34:2795–802. doi: 10.1128/jcm.34.11.2795-2802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Titus RG, Muller I, Kimsey P, Cerny A, Behin R, Zinkernagel RM, et al. Exacerbation of experimental murine cutaneous leishmaniasis with CD4+ Leishmania major-specific T cell lines or clones which secrete interferon-gamma and mediate parasite-specific delayed-type hypersensitivity. Eur J Immunol. 1991;21:559–67. doi: 10.1002/eji.1830210305. [DOI] [PubMed] [Google Scholar]
- 33.Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–55. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 34.Spath GF, Epstein L, Leader B, Singer SM, Avila HA, Turco SJ, et al. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A. 2000;97:9258–63. doi: 10.1073/pnas.160257897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–42. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One. 2009;4:e4801. doi: 10.1371/journal.pone.0004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramsey AJ, Daubner SC, Ehrlich JI, Fitzpatrick PF. Identification of iron ligands in tyrosine hydroxylase by mutagenesis of conserved histidinyl residues. Protein Sci. 1995;4:2082–6. doi: 10.1002/pro.5560041013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erlandsen H, Bjorgo E, Flatmark T, Stevens RC. Crystal structure and site-specific mutagenesis of pterin-bound human phenylalanine hydroxylase. Biochemistry. 2000;39:2208–17. doi: 10.1021/bi992531+. [DOI] [PubMed] [Google Scholar]
- 39.Dickson PW, Jennings IG, Cotton RG. Delineation of the catalytic core of phenylalanine hydroxylase and identification of glutamate 286 as a critical residue for pterin function. J Biol Chem. 1994;269:20369–75. [PubMed] [Google Scholar]
- 40.Jiang GC, Yohrling GJt, Schmitt JD, Vrana KE. Identification of substrate orienting and phosphorylation sites within tryptophan hydroxylase using homology-based molecular modeling. J Mol Biol. 2000;302:1005–17. doi: 10.1006/jmbi.2000.4097. [DOI] [PubMed] [Google Scholar]
- 41.Daubner SC, Melendez J, Fitzpatrick PF. Reversing the substrate specificities of phenylalanine and tyrosine hydroxylase: aspartate 425 of tyrosine hydroxylase is essential for L-DOPA formation. Biochemistry. 2000;39:9652–61. doi: 10.1021/bi000493k. [DOI] [PubMed] [Google Scholar]
- 42.Liang XH, Haritan A, Uliel S, Michaeli S. trans and cis splicing in trypanosomatids: mechanism, factors, and regulation. Eukaryot Cell. 2003;2:830–40. doi: 10.1128/EC.2.5.830-840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steiger RF, Steiger E. Cultivation of Leishmania donovani and Leishmania braziliensis in defined media: nutritional requirements. J Protozool. 1977;24:437–41. doi: 10.1111/j.1550-7408.1977.tb04771.x. [DOI] [PubMed] [Google Scholar]
- 44.Cunningham ML, Titus RG, Turco SJ, Beverley SM. Regulation of differentiation to the infective stage of the protozoan parasite Leishmania major by tetrahydrobiopterin. Science. 2001;292:285–7. doi: 10.1126/science.1057740. [DOI] [PubMed] [Google Scholar]
- 45.Nosanchuk JD, Casadevall A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob Agents Chemother. 2006;50:3519–28. doi: 10.1128/AAC.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christensen BM, Li J, Chen CC, Nappi AJ. Melanization immune responses in mosquito vectors. Trends Parasitol. 2005;21:192–9. doi: 10.1016/j.pt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Siltberg-Liberles J, Steen IH, Svebak RM, Martinez A. The phylogeny of the aromatic amino acid hydroxylases revisited by characterizing phenylalanine hydroxylase from Dictyostelium discoideum. Gene. 2008;427:86–92. doi: 10.1016/j.gene.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Plonka PM, Grabacka M. Melanin synthesis in microorganisms--biotechnological and medical aspects. Acta Biochim Pol. 2006;53:429–43. [PubMed] [Google Scholar]
- 49.Casadevall A, Rosas AL, Nosanchuk JD. Melanin and virulence in Cryptococcus neoformans. Curr Opin Microbiol. 2000;3:354–8. doi: 10.1016/s1369-5274(00)00103-x. [DOI] [PubMed] [Google Scholar]
- 50.Sienkiewicz N, Ong HB, Fairlamb AH. Trypanosoma brucei pteridine reductase 1 is essential for survival in vitro and for virulence in mice. Mol Microbiol. 2010 doi: 10.1111/j.1365-2958.2010.07236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steventon GB, Mitchell SC. Phenylalanine 4-monooxygenase and the role of endobiotic metabolism enzymes in xenobiotic biotransformation. Expert Opin Drug Metab Toxicol. 2009;5:1213–21. doi: 10.1517/17425250903179318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genomic DNA isolated from L. major FV1 was digested with the indicated panel of restriction endonuclease, and subjected to Southern blot analysis using a radiolabeled LmjPAH coding sequence. The sizes of molecular weight standards (kb) are shown.
Leishmania WT and KO cells were grown individually under ‘standard/high’ or ‘minimal/low’ concentrations of H4B (H, 2, and L, 0.001 μg/ml), epinephrine (EP; 100 μM), norepinephrine (NP; 100 μM) or propanolol (PPL, 10 μM) as described in the text, and the percent of metacyclics was measured after 3 days in stationary phase. The average and standard deviation of duplicate samples from a representative experiment is shown.
Evolutionary relationships amongst the indicated AAAH were calculated using the ClustalW algorithm [65] included in the Lasergene package (DNAStar, Inc.). Where known, the aromatic amino acid specificity is indicated by the one-letter amino acid code. D. melanogaster PheH/TrpH (P17276) and TyrH (NP_476897); C. elegans PheH (P90925), TrpH (NP_495584), and TyrH (NP_871903); Human TyrH (P07101), TrpH (P17752) and TyrH (P00439); Leishmania major PAH (AY273788); L. infantum (XP_001470169); L. braziliensis (XP_001566169); Bodo saltans (ACI15907); Toxoplasma gondii 2 (ACB99414); Dictyostelium discoideum (XP_641959); Pseudomonas (NP_249563); Chromobacterium (NP_902850); and Caulobacter (NP_420423).