Abstract
Toll-like receptors (TLRs) are involved in the induction of an adequate immune response on infection. We hypothesized that genetic variation in TLR4 and TLR2 genes could influence this response and lead to variability in cytokine production and survival. We tested this hypothesis in 4292 participants who were followed up for all-cause mortality for 6 years and live under adverse environmental conditions in the Upper-East region of Ghana, where malaria is endemic. In 605 participants, tumor necrosis factor-α and interleukin-10 (IL10) production, after stimulation with lipopolysaccharide and zymosan, was measured. In addition, 34 single-nucleotide polymorphisms (SNPs) in TLR4 and 12 SNPs in TLR2 were genotyped and tested for association with cytokine production, malaria infection and mortality. In this comprehensive gene-wide approach, we identified novel SNPs in the TLR4 gene that influence cytokine production. From the analyzed SNPs, rs7860896 associated the strongest with IL10 production (P=0.0005). None of the SNPs in this study associated with malaria or overall mortality risks. In conclusion, we demonstrate that genetic variation within the TLR4 gene influences cytokine production capacity, but in an endemic area does not influence the susceptibility to malaria infection or mortality.
Keywords: TLR2, TLR4, polymorphisms, mortality, cytokines, Plasmodium falciparum
Introduction
Pathogen recognition receptors are of crucial importance for survival in an environment with high infectious pressure. Toll-like receptor 4 (TLR4) and TLR2 are evolutionarily conserved pathogen recognition receptors1 that mediate the NF-κB pathway, which results in an induction of an immune response downstream. TLR4 is mainly involved in the recognition of Gram-negative bacteria by lipopolysaccharide (LPS),2 and also pathogen-associated molecular patterns from mycobacteria, Plasmodium,3 or viruses.4 On the other hand, TLR2 is involved in the recognition of Gram-positive bacteria, mycobacteria,5 viruses, fungi and parasites.4 Both TLRs belong to the first line of defense and are essential for the induction of an adequate innate immune response on infection. To date, several studies have associated genetic variation in TLR4 and TLR2 genes with multiple immune-mediated diseases,6 suggesting that they influence immune responses on infections and consequently survival probabilities.
Over the years, several polymorphisms in TLR genes that influence endotoxin responsiveness7, 8 and susceptibility to disease have been identified.6 In TLR4, the most extensively studied genetic variants are Asp299Gly and Thr399Ile, which have been associated with LPS hyporesponsiveness7 and pathogenesis of malaria,3 Gram-negative sepsis,9 and atherosclerosis.10 Asp299Gly is more frequent in Africa, whereas in co-segregation with Thr399Ile, it was found to be more frequent in Europe.11 Furthermore, in the TLR2 gene, genetic variation was associated with a poor cell-mediated immune response in patients with the lepromatous form of leprosy.12, 13 The studied variants represent only a small fraction of the genetic variation present in TLR4 and TLR2 genes. To date, no systematic evaluation of common variants in these genes on induction of innate immune responses and survival has been conducted.
In this study, we undertook a comprehensive gene-wide association analysis of genetic variation in TLR4 and TLR2 genes, in relation to cytokine production induced by respective endotoxins, Plasmodium falciparum susceptibility and survival. The study was carried out in a large contemporary rural population in the Upper-East region of Ghana living under adverse environmental conditions characterized by high infectious pressure.
Materials and methods
Study population
This study was conducted in a rural area in Garu-Tempane district in the Upper-East region of Ghana. The area is poor and mortality rates are high, with the main causes of death being malaria, diarrhea and poor nutrition.14, 15 Inhabitants belong to different ethnic groups such as Bimoba, Fulani, Busanga, Kusasi and Maprusi. Surveys on demographics were held annually from June to August, starting from 2002,16 in which population information including births, deaths and migration was updated. Each year, mouth swabs for DNA analyses were obtained from a subset of adults and newborns. In 2006, a series of whole-blood assays were carried out on a subset of the population, consisting of women of all ages, children and elderly. In 2008, venous blood samples were collected using EDTA-containing vials from a different but similar subset of the population to test for P. falciparum DNA. Subsets were chosen randomly from the total study population, representing all age categories. The population was followed up for survival until 2008. All procedures in this study have been approved by the Leiden University Medical Center, by the district health officers of the Upper-East Region and by the Medical Ethical Committee of the Ghanaian Ministry of Health. Informed consent was obtained from all participants by oral translation of the research purposes and procedures into the local language. As illiteracy rates were high, thumbprints were given for approval.
Whole-blood and cytokine assays
Whole-blood assays were performed as described elsewhere.17, 18 In brief, 4 ml venous blood was collected in the morning in a sterile endotoxin-free lithium heparin tube (Greiner BioOne GmbH, Kremsmünster, Austria) and was suspended at a dilution of 1:1 with RPMI 1640 medium supplemented with 25 m of Hepes buffer and -glutamine (Gibco, Breda, The Netherlands) containing 10 000 IU/ml penicillin and 10 000 μg/ml streptomycin (cat. no. 15140-122, Invitrogen, Breda, The Netherlands). Blood was incubated with 10 μg/ml Escherichia coli LPS (a TLR4 ligand2) (0111:B4 L2630, phenol extracted, Sigma-Aldrich, Zwijndrecht, The Netherlands) and 100 μg/ml Saccharomyces cerevisiea zymosan A (a TLR2 ligand19) (cat. no. Z4250, Sigma, Schnelldorf, Germany). Cytokine-enriched supernatants were obtained by culturing cells for 24 h at 37 °C in a humidified atmosphere containing CO2. To create the right atmosphere for incubation, the cell culture plates were placed in a tightly closed plastic container with drops of water and a burning candle inside.20 Pretesting of this method in five staff members in Ghana and Dutch study sites revealed a high correlation of TNFα and IL10 production after the candle method and the original method, in which an incubator is set at 5% CO2 (data not shown). After incubating, the solutions were centrifuged. Frozen supernatants were transported on dry ice to the Netherlands for further analysis.
TNFα and IL10 cytokine concentrations were measured in the Netherlands with ELISA kits (PeliKine Compact Sanquin Reagents, Amsterdam, The Netherlands), according to the manufacturer's guidelines. Each sample was assessed in duplicate.
SNP selection and genotyping
SNPs from the TLR4 (chr9: 117,538,161–117,566,709) and TLR2 (chr4: 154,956,754–154,994,131) gene regions were selected from the HapMap database release #21 (http://www.hapmap.org) using the Yoruba in Ibadan, Nigeria (Yoruba) data. The Haploview's program Tagger21 was used to derive a set of tag SNPs from the whole-gene region, such that each common SNP (≥5%) in that set had a value of r2≥0.8. Besides the SNPs obtained through this approach, polymorphisms that were shown to be functional and/or were associated with a phenotype in any population were included. All SNPs were genotyped using mass spectrometry (Sequenom Inc., San Diego, CA, USA), according to the manufacturer's instructions. In total, 34 SNPs in TLR4 and 12 SNPs in TLR2 genes were genotyped successfully.
P. falciparum PCR
Specific P. falciparum DNA was detected in a multiplex real-time PCR format as described before,22, 23 with some modifications. The assay was designed for the detection of all four human Plasmodium species, including the amplification and detection of a nonrelated DNA internal control. DNA isolation and setup of PCRs were performed using a custom-made Hamilton robot platform. DNA was isolated from 200 μl blood with QIAamp DNA-easy 96-well plates (Qiagen, Venlo, The Netherlands), according to the manufacturer's recommendations. Plasmodium-specific primers and Plasmodium species-specific minor groove binding TaqMan probes (Applied Biosystems, Foster City, CA, USA) based on an SSU RNA gene target were used to amplify and detect Plasmodium species-specific products of ∼150 bp.22, 23 Amplification consisted of 15 min at 95°C, followed by 50 cycles of 15 s at 95°C, 30 s at 60°C and 30 s at 72°C. Negative and positive control samples were included in each amplification run. Amplification, detection and analysis were performed using the CFX real-time detection system (Bio-Rad Laboratories, Veenendaal, The Netherlands). The cycle threshold (Ct) value for the P. falciparum-specific fluorescent label was used as output, reflecting the parasite-specific DNA load.
Statistical analyses
The program Haploview24 was used to estimate allele frequencies and to estimate pair-wise linkage disequilibrium (LD). The program PLINK25 (Center for Human Genetic Research, Massachusetts General Hospital, Boston, MA, USA) was used to test for Hardy–Weinberg equilibrium. Haplotypes and haplotype frequencies were calculated using the program Phase.26 In all haplotype analyses, the posterior probabilities of pairs of haplotypes per participant, as estimated by Phase, were used as weights. As the TNFα and IL10 levels were nonnormally distributed, z-scores were calculated on log-transformed data. The association between cytokine production P. falciparum Ct value and SNPs or haplotypes was analyzed using linear regression. Logistic regression was used for analyses of malaria positivity and Cox proportional hazards model for calculating mortality risks. All analyses were adjusted for possible confounders such as age, sex and tribe. Additional confounder in this population is compound wealth, as it has been shown to affect mortality,15 and possibly disease pathogen exposure. In all analyses, an additive model was used, assuming a linear association of each additional SNP allele with the outcome. All analyses were performed with STATA version 9 (StataCorp LP, College Station, TX, USA) statistical software.
Results
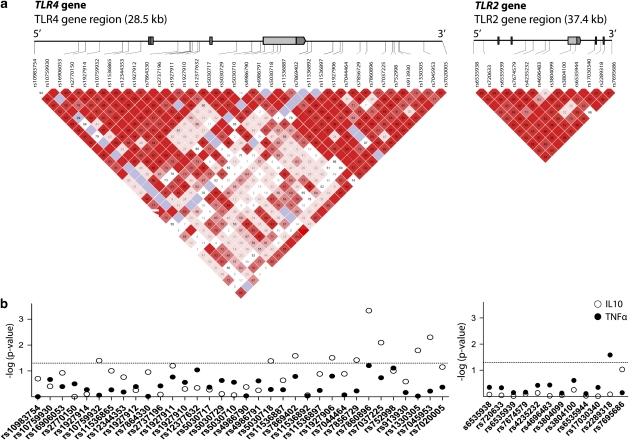
Table 1 provides the characteristics of the study population. From the total population, DNA was available from 4292 participants. In the TLR4 and TLR2 genes, 34 SNPs and 12 SNPs, respectively, were genotyped (Figure 1a; Supplementary Tables 1, 2). All SNPs were in Hardy–Weinberg equilibrium in newborns, except the rs3804099 SNP in the TLR2 gene (P-value=0.035). Minor allele frequencies of SNPs ranged from 0.005 to 0.471. The Thr399Ile variant was very rare in this population (minor allele frequency=0.012). This was in accordance with another African population (Yoruba in HapMap).
Table 1. Characteristics of the study population.
| n=4292 | |
|---|---|
| Age range (years) | 0–94 |
| Median age | 35 |
| Female (%) | 2932 (68) |
| Follow-up years (mean, SD) | 4.40 (0.90) |
| Tribe (%) | |
| Bimoba | 72.0 |
| Kusasi | 21.6 |
| Other | 6.4 |
| Mortality during follow-up | |
| All (n, %) | 292 (6.8) |
| <5 years (n, %) | 77 (8.0) |
| IL10 (pg/ml) (median, IQR) | |
| LPS (n=605) | 13 065 (8092–19 714) |
| Zymosan (n=604) | 13 032 (8844–18 673) |
| TNFα (pg/ml) (median, IQR) | |
| LPS (n=605) | 4468 (3212–5960) |
| Zymosan (n=604) | 227 (128–398) |
| Plasmodium falciparum (n=647) | |
| Positives (n, %) | 554 (85.6) |
| Median Ct value (IQR) (n=554) | 30.7 (28.3–33.1) |
Abbreviations: IL10, interleukin-10; IQR, interquartile range; LPS, lipopolysaccharide; SD, standard deviation; TNFα, tumor necrosis factor-α.
Figure 1.
TLR4 and TLR2 gene structure and association with cytokine production. (a) The TLR4 (chromosome 9) and TLR2 (chromosome 4) genes cover 28.5 and 37.4 kb, respectively. The location of the genotyped SNPs is indicated with vertical lines. Pair-wise linkage disequilibrium (LD) (D′) as observed in the Ghanaian research population (n=4292) is also depicted. (b) Association between cytokine production and tagging SNPs in TLR4 and TLR2 from a Ghanaian population. Data presented as –log P-values for the association between SNPs in TLR4 and TLR2 genes and TNFα (closed circles) and IL10 (open circles) production (n=605), as obtained by linear regression adjusted for age, sex compound wealth and tribe. Cytokine production was induced by 24-h whole-blood stimulation with Escherichia coli LPS (TLR4) and Saccharomyces cerevisiea zymosan (TLR2). The horizontal dotted line indicates the 0.05 P-value threshold.
Cytokine levels were measured ex vivo in 605 participants after stimulation with LPS (a ligand for TLR4) and zymosan (a ligand for TLR2). Association analyses between SNPs in the TLR4 and TLR2 genes showed genetic variants at the 3′-UTR of the TLR4 gene that significantly associate with LPS-induced IL10 production (Figure 1b, Supplementary Table 3). In particular, SNP rs7860896 was highly associated with IL10 production (P=0.0005). For the TLR4 (Asp299Gly and Thr399Ile) variants that have been reported earlier in the literature, no associations with ex vivo-stimulated cytokine production were observed. In the TLR2 gene, only the rs2289318 SNP associated with cytokine production. Carriers of this variant produce less TNFα than do noncarriers (P=0.026).
As the SNPs at the 3′ end of the TLR4 gene are in LD (Figure 1a), we estimated haplotypes (SNPs ranging from rs1927906 to rs7045953) and tested their association with cytokine production. When compared with haplotype 1, which is the most frequent (0.396) and carries no variant alleles, h2, h3 and h6 haplotypes were significantly associated with increased IL10 production. Furthermore, haplotype h6 was also associated with increased TNFα production (Table 2). None of these haplotypes were in strong LD with variants Asp299Gly and Thr399Ile (R2=0.026). Haplotypes were also estimated for the TLR2 gene. However, analyses with TLR2 haplotypes revealed no associations with cytokine production (Table 2, Supplementary Table 4).
Table 2. Associations between haplotypes in TLR2 and TLR4 genes and cytokine production.
| IL10 | TNFα | |||||
|---|---|---|---|---|---|---|
| Haplotype | Frequency | Estimate (SE) | P-value | Estimate (SE) | P-value | |
| TLR4 | ||||||
| H1 | GTGATTTAA | 0.396 | Reference | Reference | ||
| H2 | AATGTTTAG | 0.133 | 0.18 (0.08) | 0.035 | 0.03 (0.09) | 0.71 |
| H3 | GTTGTTTAG | 0.094 | 0.24 (0.09) | 0.010 | 0.12 (0.10) | 0.19 |
| H4 | ATGGCGCAA | 0.093 | 0.19 (0.10) | 0.068 | 0.10 (0.10) | 0.32 |
| H5 | ATGATTTAA | 0.063 | 0.01 (0.15) | 0.94 | 0.06 (0.12) | 0.63 |
| H6 | ATGGCGTAA | 0.055 | 0.21 (0.10) | 0.038 | 0.25 (0.11) | 0.024 |
| TLR2 | ||||||
| H1 | CCCTTCTTCGGA | 0.195 | Reference | Reference | ||
| H2 | CCCTTCCTCGCA | 0.133 | −0.03 (0.10) | 0.78 | −0.15 (0.09) | 0.10 |
| H3 | CCCTTTCTCAGA | 0.125 | 0.00 (0.10) | 0.99 | 0.03 (0.10) | 0.76 |
| H4 | TTCTTTCTCGGA | 0.107 | 0.01 (0.10) | 0.92 | −0.08 (0.10) | 0.45 |
| H5 | CCTGGTTTTGGA | 0.097 | −0.01 (0.12) | 0.91 | −0.10 (0.12) | 0.40 |
| H6 | CCCTTCCTCGCG | 0.073 | 0.14 (0.11) | 0.22 | −0.10 (0.10) | 0.32 |
| H7 | CCCTTCTTCAGA | 0.054 | 0.03 (0.13) | 0.83 | −0.10 (0.15) | 0.51 |
Abbreviation: SE, standard error.
Cytokines were induced by E. coli LPS stimulation for 24 h. Haplotypes included SNPs (from 5' to 3') rs1927906 to rs7045953.
Linear regression was adjusted for age, sex, compound wealth and tribe. The estimate of cytokine production is expressed as z-scores with standard error, which indicate the increase in cytokine production per copy of the haplotype.
We next tested whether haplotypes in TLR4 and TLR2 genes influence susceptibility to malaria and survival. In TLR4, we found that haplotype h3 associated with a lower Ct value for malaria, indicating a higher load of malaria parasites. Carriers of this haplotype did not have a higher prevalence of malaria. In TLR2, we found no significant associations with P. falciparum load or prevalence (Table 3, Supplementary Tables 5, 6). During the 6 years of follow-up, 292 (6.8%) participants died, of whom 77 (8.0%) were children younger than 5 years of age. None of the haplotypes in TLR4 or TLR2 were significantly associated with all-cause mortality (Table 3, Supplementary Tables 7 and 8).
Table 3. Associations between haplotypes in TLR2 and TLR4 genes, Plasmodium falciparum susceptibility and mortality.
| P. falciparum | Mortality | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | Ct value (SE) | P-value | HR | 95% CI | |
| TLR4 | ||||||
| H1 | Reference | Reference | Reference | |||
| H2 | 0.89 | 0.52–1.52 | −0.48 (0.29) | 0.10 | 1.17 | 0.91–1.49 |
| H3 | 1.34 | 0.67–2.68 | −0.61 (0.31) | 0.050 | 1.04 | 0.80–1.34 |
| H4 | 0.98 | 0.56–1.71 | −0.64 (0.34) | 0.059 | 0.88 | 0.66–1.16 |
| H5 | 1.15 | 0.51–2.59 | −0.27 (0.43) | 0.54 | 0.92 | 0.66–1.30 |
| H6 | 1.51 | 0.66–3.43 | 0.00 (0.37) | 1.00 | 0.98 | 0.68–1.40 |
| TLR2 | ||||||
| H1 | Reference | Reference | Reference | |||
| H2 | 0.81 | 0.46–1.45 | −0.13 (0.32) | 0.69 | 1.04 | 0.80–1.36 |
| H3 | 0.98 | 0.51–1.87 | 0.22 (0.33) | 0.51 | 1.01 | 0.76–1.33 |
| H4 | 0.67 | 0.35–1.27 | −0.34 (0.38) | 0.37 | 0.95 | 0.70–1.29 |
| H5 | 1.16 | 0.57–2.36 | −0.45 (0.37) | 0.23 | 0.97 | 0.71–1.33 |
| H6 | 0.94 | 0.42–2.13 | 0.82 (0.46) | 0.077 | 0.96 | 0.69–1.34 |
| H7 | 0.90 | 0.40–2.01 | 0.11 (0.42) | 0.79 | 0.96 | 0.68–1.37 |
Abbreviations: HR, hazard ratio; OR, odds ratio; SE, standard error.
Linear, logistic and Cox regression were adjusted for age, sex, compound wealth and tribe. The estimate of cytokine production is expressed as z-scores with SE, which indicate the increase in cytokine production per copy of the haplotype.
Discussion
In this study, we tested whether genetic variation in TLR4 and TLR2 genes influences innate immune responses, susceptibility to malaria and survival in a population living under high infectious pressure. The comprehensive gene-wide association approach revealed novel genetic variants at the 3′ end of the TLR4 gene that contributed to higher IL10 production. Similar associations were observed for three different TLR4 haplotypes. One haplotype in TLR4 was associated with malaria susceptibility. However, none of the SNPs or haplotypes influenced all-cause mortality risks in this population. Similarly, we found no associations with cytokine production, malaria susceptibility or mortality with TLR2 SNPs, nor with the most commonly studied variants, Asp299Gly and Thr399Ile SNPs, in the TLR4 gene. These variants were very rare in our study population (MAFs: 0.075 and 0.012, respectively), as in other African populations. Moreover, these variants were not in LD with the haplotypes that associated with cytokine production. From all analyzed SNPs, rs7860896 showed the strongest association with IL10 production, suggesting a functional relevance. It is interesting that rs7860896 has a very high frequency in African (46%) population compared with Caucasian (6%) or Asian (1%) populations (dbSNP data). As TLR4 and TLR2 are involved in the initiation of the immune response to a broad spectrum of pathogens, it has been proposed that local evolutionary pressures by infections may have led to differences in TLR4 polymorphisms in various populations.11
Using a tagging SNP approach, we identified a genetic variation located in the 3′-UTR region of the TLR4 gene that associated with cytokine production. The majority of genetic variants in the TLR4 gene are located in the extracellular domain, which is required for pathogen recognition, rather than in the cytoplasmic domain,27 which is required for anchoring and the induction of further pathways downstream via MyD88 or TRIF.28 As there were no genes near the 3′-UTR of the TLR4 gene, or in LD with any functional variant in the extracellular domain, our results might be an indication of functional variation present in the 3′-UTR region itself. As this region is a noncoding region, further research has to reveal whether this might be a binding site for miRNA,29 regulating expression at a posttranscriptional level.
A higher anti-inflammatory response has previously been shown to be associated with susceptibility to meningococcal disease.30 On the other hand, anti-inflammatory responses are also suggested to be protective against further stages of diseases, in which downregulation of an excessive proinflammatory response is crucial for survival.31 This might also explain the contradictory findings that were reported with regard to TLR4 polymorphisms. For example, the Asp299Gly variant in the TLR4 gene was associated with higher TNFα production and protection against malaria infection,11 and also with susceptibility to severe malaria,3 resistance to infection with Gram-negative bacteria such as Legionella32 and with further progression of Gram-negative infections as observed in sepsis.9, 33 This suggests that the reported TLR4 variants provide protection on initial infection, but in the long term, may instead contribute to a lack in downregulatory capacity. Nevertheless, this might explain our negative findings with regard to malaria susceptibility and survival in this population. Moreover, as malaria prevalence is very high in this population and TLRs solely serve as recognition and induction of immune responses, we assume that TLRs are not the main determinant of the outcome of infection.
Over evolutionary history, malaria has had a significant impact on the human genome.31 Another aspect besides infectious pressure with evolutionary consequences is the influence of the host immune response on fertility and abortion. There is vast literature indicating that proinflammatory immune responses might have beneficial consequences for survival, but might be detrimental for reproduction.34 Therefore, we expect that, with regard to TLR polymorphisms as well, selection will be balanced. Knowing cause-specific mortality in the study population would provide more insight into this mechanism. Unfortunately, collecting information on the cause of mortality and on disease history deemed impossible in this rural research population.
The strengths of this study include the high number of genotyped polymorphisms, which were selected to cover the effects of most of the genetic variation present in both genes. However, the large number of SNPs analyzed is also a limitation, as considering the number of tests performed, adjustment for multiple testing would eliminate many of the statistically significant associations observed. A limitation of the study is the lack of data on other infectious diseases and on specific mortality causes. Furthermore, we could not identify associations of TLR2 polymorphisms and cytokine production. As zymosan was used in this assay, which is known to induce, besides TLR2, Dectin-1, we consider this stimulation to be not as pure as that with other particles that induce TLR2 only. By using zymosan, additional stimulation besides TLR2 might have introduced noise. This might be an explanation that we could not identify clear associations of TLR2 SNPs and cytokine production. In this publication, we have used a single amount of 10 μg/ml LPS for TLR4 stimulation. We acknowledge that, given the inconsistency of TLR agonist doses used in relation to cytokine production in various publications, in further research, sensitivity as well as maximum capacity in association with polymorphisms might be better addressed using various doses of an agonist. Moreover, in further research, various cytokines other than IL10 and TNFα might give additional value.
In conclusion, we showed for the first time that genetic variation in the 3′-UTR region of the TLR4 gene is associated with cytokine production. Although this variation is likely to influence disease susceptibility in rural Ghana, we did not observe associations with malaria or mortality risks.
Acknowledgments
This research was supported by the Netherlands Foundation for the Advancements of Tropical Research (Grant number WOTRO 93-467), the Netherlands Organization for Scientific Research (NWO 051-14-050), the EU-funded Network of Excellence LifeSpan (FP6 036894), the Netherlands Genomics Initiative/Netherlands Organisation for Scientific Research (NWO) (050-60810) and the Stichting Dioraphte. We want to thank everybody who was part of the research team. Furthermore, we also thank Margo van Schie-Troost and Marja Kersbergen-van Oostrom for their work on cytokine assays, Dennis Kremer and Eka Suchiman for assistance in genetic studies, and Eric Brienen and Jaco Verweij for their work on the malaria PCR.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Mockenhaupt FP, Cramer JP, Hamann L, et al. Toll-like receptor (TLR) polymorphisms in African children: common TLR-4 variants predispose to severe malaria. Proc Natl Acad Sci USA. 2006;103:177–182. doi: 10.1073/pnas.0506803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misch EA, Hawn TR. Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci (Lond) 2008;114:347–360. doi: 10.1042/CS20070214. [DOI] [PubMed] [Google Scholar]
- Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- Michel O, LeVan TD, Stern D, et al. Systemic responsiveness to lipopolysaccharide and polymorphisms in the toll-like receptor 4 gene in human beings. J Allergy Clin Immunol. 2003;112:923–929. doi: 10.1016/j.jaci.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002;162:1028–1032. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- Kiechl S, Lorenz E, Reindl M, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- Ferwerda B, McCall MB, Alonso S, et al. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc Natl Acad Sci USA. 2007;104:16645–16650. doi: 10.1073/pnas.0704828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochud PY, Hawn TR, Aderem A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J Immunol. 2003;170:3451–3454. doi: 10.4049/jimmunol.170.7.3451. [DOI] [PubMed] [Google Scholar]
- Kang TJ, Chae GT. Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunol Med Microbiol. 2001;31:53–58. doi: 10.1111/j.1574-695X.2001.tb01586.x. [DOI] [PubMed] [Google Scholar]
- Meij JJ, de Craen AJ, Agana J, Plug D, Westendorp RGJ. Low-cost interventions accelerate epidemiological transition in Upper East Ghana. Trans R Soc Trop Med Hyg. 2009;103:173–178. doi: 10.1016/j.trstmh.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Van Bodegom D, May L, Kuningas M, et al. Socio-economic status by rapid appraisal is highly correlated with mortality risks in rural Africa. Trans R Soc Trop Med Hyg. 2009;103:795–800. doi: 10.1016/j.trstmh.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Ziem JB, Spannbrucker N, Magnussen P, et al. Oesophagostomum bifurcum-induced nodular pathology in a highly endemic area of Northern Ghana. Trans R Soc Trop Med Hyg. 2005;99:417–422. doi: 10.1016/j.trstmh.2004.07.008. [DOI] [PubMed] [Google Scholar]
- May L, Van Bodegom D, Kuningas M, et al. Performance of the whole-blood stimulation assay for assessing innate immune activation under field conditions. Cytokine. 2009;45:184–189. doi: 10.1016/j.cyto.2008.12.010. [DOI] [PubMed] [Google Scholar]
- van der Linden MW, Huizinga TW, Stoeken DJ, Sturk A, Westendorp RGJ. Determination of tumour necrosis factor-alpha and interleukin-10 production in a whole blood stimulation system: assessment of laboratory error and individual variation. J Immunol Methods. 1998;218:63–71. doi: 10.1016/s0022-1759(98)00108-2. [DOI] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbrink BD, Stienstra Y, Huitema MG, et al. Cytokine responses to stimulation of whole blood from patients with Buruli ulcer disease in Ghana. Clin Diagn Lab Immunol. 2005;12:125–129. doi: 10.1128/CDLI.12.1.125-129.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- Adegnika AA, Verweij JJ, Agnandji ST, et al. Microscopic and sub-microscopic Plasmodium falciparum infection, but not inflammation caused by infection, is associated with low birth weight. Am J Trop Med Hyg. 2006;75:798–803. [PubMed] [Google Scholar]
- Muller-Stover I, Verweij JJ, Hoppenheit B, Gobels K, Haussinger D, Richter J. Plasmodium malariae infection in spite of previous anti-malarial medication. Parasitol Res. 2008;102:547–550. doi: 10.1007/s00436-007-0804-4. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova I, Poltorak A, Chan EK, McBride C, Beutler B. Phylogenetic variation and polymorphism at the toll-like receptor 4 locus (TLR4) Genome Biol. 2000;1:RESEARCH002.1–002.10. doi: 10.1186/gb-2000-1-1-research002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Bi Y, Liu G, Yang R. MicroRNAs: novel regulators during the immune response. J Cell Physiol. 2009;218:467–472. doi: 10.1002/jcp.21639. [DOI] [PubMed] [Google Scholar]
- Westendorp RGJ, Langermans JA, Huizinga TW, et al. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–173. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawn TR, Verbon A, Janer M, Zhao LP, Beutler B, Aderem A. Toll-like receptor 4 polymorphisms are associated with resistance to Legionnaires' disease. Proc Natl Acad Sci USA. 2005;102:2487–2489. doi: 10.1073/pnas.0409831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnese DM, Calvano JE, Hahm SJ, et al. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of Gram-negative infections. J Infect Dis. 2002;186:1522–1525. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- Van Bodegom D, May L, Meij HJ, Westendorp RGJ. Regulation of human life histories: the role of the inflammatory host response. Ann NY Acad Sci. 2007;1100:84–97. doi: 10.1196/annals.1395.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.