Abstract
Based on results of ecological studies demonstrating that Vibrio cholerae, the etiological agent of epidemic cholera, is commensal to zooplankton, notably copepods, a simple filtration procedure was developed whereby zooplankton, most phytoplankton, and particulates >20 μm were removed from water before use. Effective deployment of this filtration procedure, from September 1999 through July 2002 in 65 villages of rural Bangladesh, of which the total population for the entire study comprised ≈133,000 individuals, yielded a 48% reduction in cholera (P < 0.005) compared with the control.
Cholera is a disease that continues to ravage developing countries and reemerges sporadically elsewhere throughout the world. According to the World Health Organization (WHO), 58 countries have officially reported cholera in 2001, with a total of 184,311 cases and 2,728 deaths (1). However, there were 293,113 cases of cholera worldwide in 1998, with 10,586 deaths. These annual figures of WHO actually represent the tip of the iceberg, because the morbidity and mortality caused by Vibrio cholerae is grossly underreported owing to surveillance difficulties and also for fear of economic and social consequences (2). In fact, several cholera endemic countries, e.g., Bangladesh, are not included in the WHO report. In 1991, after almost 100 years without cholera, outbreaks in 16 Latin American countries resulted in ≈400,000 reported cases of cholera and >4,000 reported deaths (3).
That cholera is a waterborne disease has long been known (4–6). Furthermore, surface water has been linked with transmission of cholera since the pioneering work of Snow in 1854 (7). Demonstration of the potential for water to transmit cholera was provided by Koch, who, after Pacini first described the Vibrio (8), isolated and characterized the bacterium, which he named Vibrio comma, and was able to find it in pond water used by an Indian community suffering a cholera epidemic (9).
The association of pathogenic vibrios with zooplankton was reported in 1973 by Kaneko and Colwell (10) and of V. cholerae with copepods by Huq et al. in 1983 (11). Commensal occurrence of Vibrio spp. in the copepod gut was demonstrated by Sochard et al. in 1979 (12). A few years later, preferential attachment of V. cholerae to copepod surfaces, egg cases, and the copepod oral region was reported by Huq et al. (11). Extensive data have since been accumulated showing that planktonic copepods play a major role in the multiplication, survival, and transmission of cholera (13–17). That environmental V. cholerae O1 can cause cholera has been established by molecular genetic evidence (18).
During spring and late summer in Bangladesh, phytoplankton blooms occur, followed by zooplankton, with heaviest blooms occurring in September and October (13, 19). Each year, the seasonal zooplankton blooms, in turn, are followed by cholera outbreaks (11, 13). It has been determined that a single copepod, depending on species and size, can carry up to 104 cells of V. cholerae (11, 17). Thus, a copepod bloom can result in the number of V. cholerae per ml of water comprising an infective dose, based on findings from human volunteer studies, showing that ≈104 to 106 V. cholerae O1 can produce clinical cholera (20). Patchiness in copepod distribution, often species specific in the aquatic environment (21), can result in significant variability in the number of copepods in water taken directly from a pond or river for drinking.
Village populations of Bangladesh depend on untreated surface water for household use, especially during times of flooding (22). Surface water from ponds and rivers is used by some villagers as a source of drinking water for reasons of taste, convenience, or a local belief that “quality” water is “natural,” i.e., not chemically treated (15, 22). Furthermore, with the current arsenic crisis in Bangladesh, as many as half of the wells drilled in the late 1960s as the answer to Bangladesh's severe surface water pollution problem have been found to be contaminated with arsenic in amounts that exceed 50 ppb, with some concentrations even 10 times higher in contaminated areas (23–26). According to a recent study conducted in Araihazar in Bangladesh by WHO, >30 million people are exposed to unsafe levels of arsenic in their drinking water, and 20% of the arsenic contaminated tube-well water users switched back to untreated surface water (27). In addition, studies showed that tube well water contained 104 to 106 total bacteria by acridine orange direct count (28) and a high incidence of zooplankton, as well as coliforms and other bacteria (29). These findings indicate that surface water has again become important as a source of household water and for drinking when no other safe water is available.
Although boiling water before drinking is effectively the better practice, because it will kill all waterborne pathogenic microorganisms, it is not used routinely in the villages because fuel wood in rural Bangladesh is both in very short supply and costly. Moreover, during severe flooding, which frequently occurs in Bangladesh, there are geographical areas that experience reduction in the quality of life to mere survival, when even the barest necessities are difficult to obtain and building fires to boil water is simply not possible.
It is common practice in villages in Bangladesh to use cloth, frequently a flat, unfolded piece of an old sari, to filter home-prepared drinks. In laboratory experiments employing electron microscopy, we found that inexpensive sari cloth, folded four to eight times, provides a filter of ≈20-μm mesh size, small enough to remove all zooplankton, most phytoplankton, and all V. cholerae attached to plankton and particulates >20 μm. Laboratory studies showed that sari cloth folded at least four times retained the V. cholerae cells attached to plankton, effectively removing >99% (>2 logs) of V. cholerae (30). Nylon net with a mesh size of ≈150 μm was also successfully used in this study to compare its effectiveness in preventing cholera because it has been used to remove cyclops from drinking water. Cyclops is a crustacean copepod common in Africa and carrier of the guinea worm larvae that causes dracunculiasis (31, 32).
Taking advantage of the knowledge that V. cholerae is autochthonous to the aquatic environment (28, 33) and resides in and on copepods, a simple filtration procedure was devised for rural villagers in Bangladesh to remove V. cholerae attached to plankton in environmental water. The hypothesis tested in the study reported here was that if the copepods with which V. cholerae is associated are removed by filtration from water of natural systems used for household purposes, including drinking, the occurrence of cholera will be significantly reduced. A 3-year study was designed to test this hypothesis and was carried out in Matlab, Bangladesh. The results are reported here.
Materials and Methods
Study Area.
Field trials were conducted in Matlab, Bangladesh, in collaboration with the International Centre for Diarrhoeal Disease Research, Bangladesh. Study villages were selected from 142 registered villages where untreated pond or river water is the source of household water. A typical household in the Matlab study area comprised an average of 5.6 individuals. Study families, notably the mother and other female members of the households responsible for collecting water, were trained to use the filter properly, as well as being instructed about the health significance of its application. Because Matlab, Bangladesh, where the study was undertaken, has the largest continuously operating population surveillance system in the world, established in 1963 (34), the study was able to be carried out with greater ease than would have been possible in other developing countries. Furthermore, participants in the study were guaranteed treatment by the local medical unit or hospital and all who went to the medical unit or hospital with diarrhea had stool samples tested for V. cholerae O1 and O139, which, if present in the laboratory tests, was recorded.
Before the full, extensive field trial, a pilot study was conducted to test the filtration method and determine whether compliance with the treatment protocols would be achieved. The full, extensive field trial, which included those households in the pilot study, commenced January 2001, and ran through July 2002. In both the pilot and full study, households with children under 5 years of age and using surface water for household purposes were selected for participation, because children under age 5 are at higher risk of cholera. Villages were assigned to a single treatment arm, e.g., nylon, sari, or no (control) filter. It was not feasible to assign individual households to different treatments because of the potential of families communicating with each other concerning the protocol.
Study Design.
Three groups of villages were chosen, with consideration given to education, economic, and social background, and uniform distribution among the test groups, i.e., sari filter, nylon filter, and no filter as control (Table 1). Villages with a high rate of cholera, based on the previous 3 years of data on cholera, were selected for the study. It should be noted that historic cholera rates for 1997–1999 showed no significant or even modest correlation among years (Table 2). All pair-wise correlations were <0.2 (P > 0.1). See Table 2.
Table 1.
Summary of data on populations included in the water filtration program carried out in Matlab, Bangladesh
| Pilot phase September 1999–December 2000 (16 months)
|
Full study January 2001–July 2002 (19 months)
|
|||||
|---|---|---|---|---|---|---|
| Sari | Nylon | Control | Sari | Nylon | Control | |
| No. of villages | 7 | 8 | 3 | 27 | 25 | 13 |
| No. of individuals | 11,267 | 15,418 | 18,461 | 44,429 | 44,496 | 44,194 |
| No. of individuals in the study | 4,131 | 4,477 | 4,416 | 14,709 | 15,748 | 15,662 |
| No. of households in the study | 737 | 726 | 749 | 2,750 | 2,750 | 2,750 |
Table 2.
Historical cholera rates (cases per 1,000 population): Correlation among years prior to the study
| Mean | 1997 | 1998 | 1999 | |
|---|---|---|---|---|
| 1997 | 3.54 | – | – | – |
| 1998 | 3.93 | 0.142 | – | – |
| 1999 | 1.67 | 0.052 | 0.002 | – |
Data were taken from 65 villages. Means and correlations are weighted with village population numbers serving as weights. All three reported correlations are insignificant (P > 0.25).
The pilot study comprised seven sari, eight nylon, and three control villages and covered the period September 1999–December 2000. A total of 2,212 households, with ≈740 households in each group: sari filter, nylon filter, and control, comprised a total of 45,146 people (Table 1). In addition to filters, the villagers assigned to filtration were given extensive educational information about filtering, storing, and using surface water for domestic purposes. Posters containing illustrations of plankton and bacteria were distributed, and detailed explanations were given to the villagers to emphasize the need to filter water to prevent cholera.
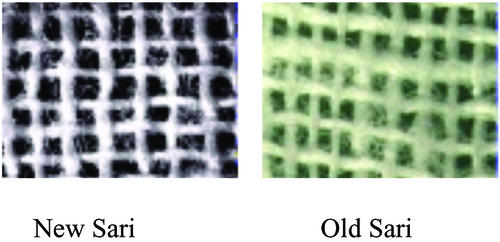
The nylon net filter was 150-μm mesh size, the same as used to control dracunculiasis in Africa, and deployed under the auspices of the WHO (31). An old sari cloth made of cotton was found to be most effective in removing V. cholerae, based on laboratory experiments (30), and was folded to produce eight layers of sari cloth that then served as a filter. After several launderings, threads of an old sari become soft and loose, reducing the pore size, compared with new sari cloth. Examination of the cloth filter by electron microscopy demonstrated an effective pore size of 20 μm when folded four to eight times (Fig. 1). The filters were placed over the neck of the water collecting pot, usually made of clay or more commonly solid brass or aluminum and locally termed “kalash” in Bangladesh. When the kalash is dipped in a pond, canal, or river, the water enters the container only by passing through the sari cloth or nylon filter. As noted above, detailed instructions were given to the villagers on how to use a filter, including emphasizing the importance of decontaminating the filters after each use. That is, after filtering, the concentrated plankton were removed first by rinsing the filter in the same river or pond water, followed by a second rinse with previously filtered water, and subsequent air drying in sunlight.
Figure 1.
Electron micrographs of a single layer of sari cloth filters. Pore size is 100–150 μm in old (laundered) sari cloth, but ≈ 20 μm if folded four to eight times.
Field Health Assistants paid a visit to each family in the study, including control groups, every 2 weeks to determine whether filtration following previously set instructions was properly followed. Instructions were clear to all groups, including control, that kalashes had to be cleaned daily, to prevent formation of biofilm.
Cholera data were obtained from the Matlab hospital records of the International Centre for Diarrhoeal Disease Research, Bangladesh. In selecting villages for the study, distance of each village to the hospital or medical care unit was approximately the same for all treatment groups. Therefore, delay or discouragement from going for treatment was not a factor in this study.
Data Forms and Questionnaires.
An extensive questionnaire was filled out by field workers during the baseline and follow-up survey. In addition, a hospital patient form for each admitted patient coming from all of the study villages was filled out by the field group supervisor. Once a cholera case was detected from any village included in this study, the field worker visited that house, an investigation was conducted to determine the source of infection, and a questionnaire was filled out, recording the information obtained.
A total of 65 villages comprised the full study, including villages of the pilot study. There were 25 villages in the nylon group, 27 in the sari group, and 13 in the control group. The control villages were generally larger in population size (Table 1). All villages in the three groups comprising the study were selected taking the distance from the Matlab Hospital into consideration so that each group was approximately at a similar distance. There were ≈44,000 individuals in each treatment, including the control. Because the overall incidence of cholera was historically relatively low (three cases per 1,000 per year is not uncommon in Matlab), a large number of participants were needed to detect changes in cholera rates.
Statistical Analyses.
Because each village was assigned a treatment, the data were analyzed with the village serving as the fundamental unit of analysis. Basic descriptive statistics for both the pilot and full study are given in Table 3. We used a generalized linear model with number of cholera cases as the response variable. The logarithm of village population size was used as an offset variable and the log of historical cholera rate and the number of months on study were used as covariates. We used a Poisson regression, but adjusted for overdispersion using the scaled deviance (35). We regarded the analysis as consisting of two preplanned comparisons, namely sari filter versus control and nylon filter versus control. We also report comparisons of sari versus nylon filter. Significance was declared at α = 0.025, so as to control study-wise error. We also calculated the effect of each filtration treatment in relation to the control using b = exp (beta). Thus, b = 0.6 for the coefficient of nylon versus control is interpreted to mean that nylon will have a cholera rate that is 60% of the control value, i.e., 40% less cholera. Similar steps were taken for the sari versus control coefficient.
Table 3.
Number of cholera cases and cholera rates before and during study
| Sari | Nylon | Control | |
|---|---|---|---|
| Pilot phase (18 villages) | |||
| 1997 | |||
| No. of cases | 68 | 99 | 63 |
| Rate | 6.15 | 6.49 | 3.50 |
| 1998 | |||
| No. of cases | 58 | 102 | 81 |
| Rate | 5.15 | 6.51 | 4.42 |
| Jan.–Aug. 1999 | |||
| No. of cases | 33 | 11 | 19 |
| Adjusted cases* | 49.5 | 16.5 | 28.5 |
| Adjusted rate | 4.34 | 1.07 | 1.54 |
| Sep. 1999–Dec. 2000 | |||
| No. of cases | 15 | 15 | 34 |
| Adjusted cases* | 11.25 | 11.25 | 25.5 |
| Adjusted rate | 1.00 | 0.73 | 1.38 |
| Full study (65 villages) | |||
| 1997 | |||
| No. of cases | 162 | 183 | 114 |
| Rate | 3.76 | 4.19 | 2.7 |
| 1998 | |||
| No. of cases | 165 | 177 | 173 |
| Rate | 3.79 | 4.01 | 4.0 |
| 1999 | |||
| No. of cases | 79 | 48 | 93 |
| Rate | 1.8 | 1.08 | 2.14 |
| 2000 | |||
| No. of cases | 22 | 38 | 32 |
| Rate | 0.50 | 0.85 | 0.724 |
| Jan. 2001–July 2002 | |||
| No. of cases | 46 | 52 | 81 |
| Adjusted cases* | 29.05 | 32.84 | 51.15 |
| Adjusted rate | 0.65 | 0.79 | 1.16 |
Cholera rates reported as no. of cases per 1,000 individuals per year. Pilot phase, September 1999–December 2000; full study, January 2001–July 2002.
Adjusted cases: 12 (No. of cases/no. of months in period).
Results and Discussion
An important finding from the pilot study was very high acceptance and compliance by the villagers of water filtration. During the pilot study, it was clearly demonstrated that cultural barriers would not prevent use of old sari cloth as a filter. Approximately 90% of the villagers filtered water in accordance with the established protocol. Only 0.6% of the population was noncompliant, i.e., did not use the filters. Of the remaining 10%, 4.2% switched to tube-well water for all uses, 2.4% relocated to another village, and 3.4% of the households could not use surface water because their ponds had gone dry during the trial period. The villagers in Bangladesh comprising the main study were in excellent compliance, far better than predicted.
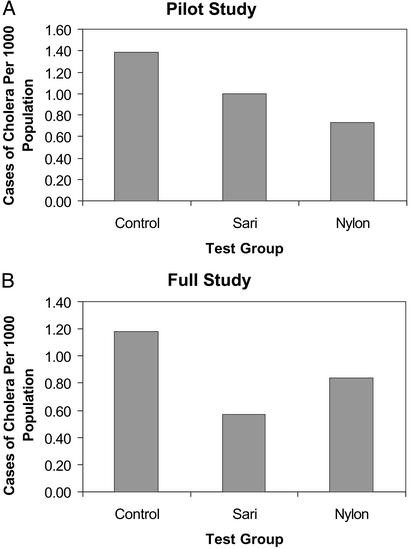
Results of the pilot study indicated that filtration reduced the number of cases of cholera when nylon net or sari cloth was used, compared with those who did not filter their water. That is, ≈38% reduction in cholera cases among filter users was achieved. However, the sample for the pilot study was too small for statistical significance. Hence, a larger study was undertaken.
In the full study, both the nylon filtration group (P < 0.02) and the sari filtration group (P < 0.005) experienced significantly lower cholera rates than the control (Fig. 2 and Table 4). Both filters were capable of removing copepods that are >150 μm in size, as well as particulate matter. In the comparison of nylon versus sari filtration, no significant difference was noted (P > 0.3) even though the sari filtration was observed to be more successful in lowering the rate of cholera. Hence, effectiveness in removing copepods was demonstrated. From the generalized linear model, we estimated that the sari group had a cholera rate ≈52% of the control, or cholera was reduced by about half. It should be noted that the years in which this study was undertaken were relatively low in cholera incidence, therefore, the cholera rates for all three study arms were low when compared with historic data (Tables 3 and 4).
Figure 2.
Comparison of cholera cases among control, sari filtration, and nylon filtration groups of villages.
Table 4.
Results of Poisson regression analysis for the full study
| df | β | SE | P | b | |
|---|---|---|---|---|---|
| Intercept | 1 | −9.4492 | 1.2787 | ||
| Nylon vs. control | 1 | −0.5153 | 0.2141 | P < 0.02 | 0.59 |
| Sari vs. control | 1 | −0.6533 | 0.2269 | P < 0.001 | 0.52 |
| Log (h2000 + 0.5) | 1 | 0.0241 | 0.1627 | NS | |
| Time on study, months | 1 | 0.1723 | 0.0687 | P < 0.02 |
Log (village size) was used as offset variable. Deviance statistic, 1.425; test statistics were adjusted for overdispersion. h2000 is the historic cholera rate for 2000. Results were similar when 1999 values were used. No significant differences were found between nylon and sari (P > 0.3).
Several conclusions can be drawn from this study, which was carried out by a highly interdisciplinary team, including sociologists, physicians, field extension agents, microbiologists, epidemiologists, ecologists, statisticians, and environmental scientists. First, significant reduction in cholera was achieved by filtering out zooplankton, namely copepods, and colonial phytoplankton from household water, both by nylon and sari filtration. From the laboratory and field studies, it was found that sari filtration removed all zooplankton, most of the phytoplankton, and all particulates >20 μm. The nylon filter removed the zooplankton, larger in size, and hence, was almost equally effective. Because cholera is dose dependent (20), by reducing the number of V. cholerae cells by filtration, we were able to achieve significant reduction in the number of cholera cases. Severity of the disease in those cases that did occur among the filtration population also appears to have been reduced. This observation will be confirmed in ongoing studies. Nylon material was also effective in filtering out copepods, but sari cloth is much less expensive, very effective, and readily available to all villagers in Bangladesh. Although sari cloth was used in Bangladesh and found to be effective, with even a visual difference in the quality of water being easily discernible, other material may be similarly functional and can be used in other parts of the world, where untreated water is used for domestic purposes and cholera is endemic. Interestingly, during the period of the study, meetings with the community health workers revealed that a large number of mothers using filtration perceived a positive decline in the incidence of diarrhea within their families. Therefore, efforts are currently being made to quantitate this finding. The importance of such a perception by a village mother would be significant in disseminating the message for effective implementation of the recommended procedure, when a filtration program is initiated. Other waterborne diarrheal diseases endemic in the Bangladesh villages are being analyzed to determine whether similar reduction was achieved and those results will be communicated separately.
In designing the full study, the study villages were not randomly selected for reasons of minimizing communication between villages. The villagers in the study used either surface water or tube-well water for drinking, but none of the villages used entirely one or the other source. Future studies will need to separate these factors to determine any effect on cholera rate. The type of storage of water in the home was not examined specifically, but will need to be addressed in future studies to determine whether the effectiveness of filtration can be improved. It was not the intention to culture water after filtration and storage in the home, because the supply of water for the household was replenished daily and instructions were given biweekly to all study households (including controls) on proper cleaning and storage of water. Visits by field workers every 2 weeks were concluded to be adequate to ensure compliance with filtration. Because each cholera case was followed up to determine probable source, in most cases the villagers had visited other, nonstudy villages where cholera had occurred. Thus, it was concluded that the biweekly field worker visits were effective. To prove this statistically, however, will require a separate, far more extensive study. Nonetheless, these factors will be addressed in future studies to improve the effectiveness of filtration and to address limitations of the research reported here.
Based on results of the 3-year study reported here, we suggest that a simple solution to a global problem can be achieved when the ecological basis of the disease transmission and its reservoir are known. In the case of cholera, ecology, climate, and environmental conditions determine the annual incidence of the disease (6, 36). Armed with this information and employing a simple preventive measure, namely filtration, we will be able to abate the disease in countries where the populace does not have water treatment and distribution facilities available. Furthermore, because climate factors have recently been shown to influence the annual occurrence of cholera, an early warning system for this disease, by incorporating environmental signals, such as El Niño, and monitoring by remote sensing is feasible for cholera endemic regions of the world (6, 36–38).
Acknowledgments
We acknowledge the assistance of Adam Frederick with the electron microscopy. This work was supported by National Institute of Nursing Research of the National Institutes of Health Grant RO1 NRO427-01A1.
Abbreviation
- WHO
World Health Organization
References
- 1.World Health Organization. Wkly Epidemiol Rec. 2002;77:257–268. [Google Scholar]
- 2.Sack D A. Bull Inst Pasteur. 1995;93:229–235. [Google Scholar]
- 3.World Health Organization. Wkly Epidemiol Rec. 2000;31:249–256. [Google Scholar]
- 4.Wendt E C. A Treatise on Asciatic Cholera. New York: William Wood; 1885. [Google Scholar]
- 5.Pollitzer R. Cholera. Geneva: World Health Organization; 1959. pp. 11–50. [Google Scholar]
- 6.Colwell R R. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 7.Sheppard D A E. John Snow: Anesthetist to a Queen and Epidemiologist to a Nation: A Bibliography. Cornwell Edward Island, Canada: York Point; 1995. [Google Scholar]
- 8.Pacini F. Gaz Med Italiana. 1854;6:405–412. [Google Scholar]
- 9.Koch R. Br Med J. 1884;2:403–407. doi: 10.1136/bmj.2.1235.403. , 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneko T, Colwell R R. J Bacteriol. 1973;113:24–32. doi: 10.1128/jb.113.1.24-32.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huq A, Small E B, West P A, Huq M I, Rahman R, Colwell R R. Appl Environ Microbiol. 1983;45:275–283. doi: 10.1128/aem.45.1.275-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sochard, Wilson M R, Austin B, Colwell R R. Appl Environ Microbiol. 1979;37:750–759. doi: 10.1128/aem.37.4.750-759.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huq A, Colwell R R, Rahman R, Ali A, Chowdhury M A R, Parveen S, Sack D A, Russek-Cohen E. Appl Environ Microbiol. 1990;56:2370–2373. doi: 10.1128/aem.56.8.2370-2373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamplin M, Gauzens A, Huq A, Sack D, Colwell R. Appl Environ Microbiol. 1990;56:1977–1980. doi: 10.1128/aem.56.6.1977-1980.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colwell R R, Huq A. In: Vibrio cholerae and Cholera: Molecular to Global Perspectives. Wachsmuth I K, Olsvik O, Blake P A, editors. Washington, DC: Am. Soc. Microbiol.; 1994. , Ch. 9, pp. 117–133. [Google Scholar]
- 16.Heidelberg J F, Heidelberg K B, Colwell R R. Appl Environ Microbiol. 2002;68:5488–5497. doi: 10.1128/AEM.68.11.5488-5497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidelberg J F, Heidelberg K B, Colwell R R. Appl Environ Microbiol. 2002;68:5498–5507. doi: 10.1128/AEM.68.11.5498-5507.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zo Y-G, Rivera I N G, Russek-Cohen E, Islam M S, Siddique A K, Yunus M, Sack R B, Huq A, Colwell R R. Proc Natl Acad Sci USA. 2002;99:12409–12414. doi: 10.1073/pnas.192426499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam M S, Drasar B S, Sack R B. J Diarrh Dis Res. 1994;12:245–256. [PubMed] [Google Scholar]
- 20.Cash R A, Music S I, Libonati J P, Snyder M J, Wenzel R P, Hornick R B. J Infect Dis. 1974;129:45–52. doi: 10.1093/infdis/129.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Benfield M C, Wiebe P H, Stanton T K, Davis C S, Gallager S M, Greene C H. Deep-Sea Res II. 1998;45:1175–1199. [Google Scholar]
- 22.Briscoe J. Am J Clin Nutr. 1978;31:2100–2113. doi: 10.1093/ajcn/31.11.2100. [DOI] [PubMed] [Google Scholar]
- 23.Lepkowski W. Chem Eng News. 1998;76(46):27–29. [Google Scholar]
- 24.Smith A H, Lingas E O, Rahman M. WHO Bull. 2000;78:1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes J M, Boyce J M, Levine R J, Khan M, Aziz K M A, Huq M I, Curlin G T. WHO Bull. 1982;60:395–404. [PMC free article] [PubMed] [Google Scholar]
- 26.van Geen A, Ahsan H, Horneman A H, Dhar R K, Zheng Y, Hussain I, Ahmed K M, Gelman A, Stute M, Simpson H J, et al. WHO Bull. 2002;80:732–737. [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. WHO Bull. 2002;10:2000. [Google Scholar]
- 28.Colwell R R, Tamplin M L, Brayton P R, Gauzens A L, Tall B D, Harrington D, Levine M M, Hall S, Huq A, Sack D A. In: Advances in Research on Cholera and Related Diarrhoeas. 7th Ed. Sack R B, Zinnaka Y, editors. Tokyo: K.T.K. Scientific; 1990. pp. 327–43. [Google Scholar]
- 29.Islam M S, Huq A, Siddika A, Khan M N H, Golder M M, Siddique M A, Kabir A N M H, Colwell R R. Appl Environ Microbiol. 2001;67:3328–3330. doi: 10.1128/AEM.67.7.3328-3330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huq A, Xu B, Chowdhury M A R, Montilla R, Colwell R R. Appl Environ Microbiol. 1996;62:2508–2512. doi: 10.1128/aem.62.7.2508-2512.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yohalem D, Fry S, editors. Orientation to Guinea Worm Disease: A Guide for Use in Pre-Service and In-Service Training. Arlington, VA: Water and Sanitation Health; 1991. , WASH Field Report 320. [Google Scholar]
- 32.World Health Organization. Guidelines for Cholera Control. Geneva: World Health Organization; 1993. [Google Scholar]
- 33.Grimes D J, Brayton P, Colwell R R, Gruber S H. Syst Appl Microbiol. 1985;6:221–226. [Google Scholar]
- 34.Aziz K M A, Moseley W H. In: Matlab: Women, Children and Health. Fauveau V, editor. Bangladesh, Dhaka, Bangladesh: International Centre for Diarrhoeal Disease; 1994. , Ch. 4, pp. 51–64. [Google Scholar]
- 35.Stokes M E, Davis C S, Koch G G. Categorical Data Analysis Using the SAS System. 2nd Ed. Cary, NC: SAS Institute; 2000. [Google Scholar]
- 36.Lobitz B, Beck L, Huq A, Wood B, Fuchs G, Faruque A S G, Colwell R. Proc Natl Acad Sci USA. 2000;97:1438–1443. doi: 10.1073/pnas.97.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodó X, Pascual M, Fuchs G, Faruque A S G. Proc Natl Acad Sci USA. 2002;99:12901–12906. doi: 10.1073/pnas.182203999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipp E K, Huq A, Colwell R R. Clin Microbiol Rev. 2002;15:757–770. doi: 10.1128/CMR.15.4.757-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]