Abstract
The ventral hippocampus (VH) plays critical roles in cue-induced and cocaine-primed reinstatement of cocaine seeking (Rogers and See, 2007). Subregions of the VH make distinct projections to elements of the brain relapse circuitry that mediate drug context-induced reinstatement. Thus, the VH may also critically contribute to this form of cocaine seeking in a subregion-specific manner. Accordingly, this study evaluated the hypothesis that functional inactivation of the ventral hippocampus proper (VHp) – but not of the dentate gyrus (DG) – impairs cocaine seeking elicited by re-exposure to a drug-paired environmental context. Rats were trained to lever press for un-signaled intravenous cocaine infusions (0.15 mg/infusion) in a distinct environmental context (cocaine-paired context) followed by extinction training in a distinctly different context (extinction context). Subsequently, cocaine-seeking behavior (i.e., non-reinforced active lever responding) was assessed in either the previously cocaine-paired context or the extinction context. Rats received bilateral microinfusions of the gamma-aminobutyric acid (GABA) agonist cocktail, baclofen+muscimol (BM: 1.0/.01mM), or vehicle into the VHp, DG, or the posterior dorsal hippocampus (pDH; extra-VH control) immediately before each test session. Exposure to the previously cocaine-paired context, but not the extinction context, reinstated extinguished cocaine-seeking behavior following vehicle pretreatment. BM pretreatment administered into the VHp, but not the DG or pDH, significantly attenuated drug context-induced cocaine seeking. These results indicate that the VH contributes to drug context-induced cocaine seeking in a subregion-specific manner, with the functional integrity of the VHp being necessary for memory or motivational aspects of drug-paired environmental stimuli that sustain stimulus control over goal-directed behavior.
Keywords: Cocaine, Context, Relapse, Dentate Gyrus, CA3, CA1, Functional inactivation
1.1
Over the course of chronic cocaine use, environmental stimuli repeatedly paired with the effects of the drug may acquire conditioned rewarding, conditioned reinforcing, and/or incentive motivational properties through associative learning processes (Crombag et al., 2008). As a result, exposure to drug-paired explicit stimuli or environmental contexts can elicit drug craving and seeking in former drug users, even after prolonged abstinence (Rohsenow et al., 1990; Ehrman et al., 1992; Foltin and Haney, 2000; Volkow and Fowler, 2000). Hence, identifying the neural circuitry that contributes to drug context-induced reinstatement of cocaine seeking is critical from an addiction-treatment perspective.
Converging lines of evidence from clinical and preclinical studies have revealed that addictive behaviors, including relapse to drug taking, are regulated by a mesocorticolimbic neural circuitry, which includes the hippocampal formation (Neisewander et al., 2000; Ferbinteanu and McDonald, 2001; Kalivas and McFarland, 2003; Fuchs et al., 2005; Noonan et al., 2010; Schmidt and Pierce, 2010). Consistent with this, neural activity in the hippocampus is positively correlated with self-reported measures of cue-induced drug craving (Sell et al., 2000; Kilts et al., 2001; Schneider et al., 2001; Wexler et al., 2001). In animal models of drug relapse, the dorsal hippocampus appears to play a more circumscribed role in drug context-induced cocaine-seeking behaviors (Fuchs et al., 2005), whereas the ventral hippocampus (VH) may have a broader role in regulating drug seeking. Specifically, electrical stimulation of the ventral subiculum (vSUB), an output region of the VH, is sufficient to elicit reinstatement of extinguished d-amphetamine- or cocaine-seeking behaviors (Vorel et al., 2001; Taepavarapruk and Phillips, 2003). Conversely, functional inactivation of either the VH proper (VHp; i.e. the cornu ammonis, or CA, subfields) or the vSUB impairs both explicit cue-induced and cocaine-primed cocaine-seeking behaviors (Sun and Rebec, 2003; Rogers and See, 2007), although some studies have found that the vSUB is not involved in the expression of these behaviors (Black et al., 2004). In addition, the VHp contributes to behaviors mediated by motivationally relevant contexts given that the functional integrity of the VH and vSUB are necessary for the retrieval of context-fear associations and the acquisition of context-cocaine associations, respectively (Hobin et al., 2006; Atkins et al., 2008). However, the precise involvement of the VH in instrumental drug-seeking behavior elicited by a drug-paired environmental context has not yet been established.
The subregions of the VH make unique neuroanatomical connections with one another as well as with other elements of the mesocorticolimbic reward circuitry. Early models described the VH as a trisynaptic circuit consisting of the dentate gyrus (DG) in addition to areas CA3 and CA1 of the VHp, with information entering the VH via entorhinal projections to the DG and then undergoing additional processing in CA3 and CA1 before being transmitted to subcortical structures via the vSUB (Johnston and Amaral, 1998). However, layers II and III of the entorhinal cortex (EC) also project to and can independently excite areas CA3 and CA1, respectively, and area CA3 receives collaterals from layer II neurons that enervate the DG (Steward and Scoville, 1976; Mizumori et al., 1989; Ishizuka et al., 1990; Witter, 1993; Johnston and Amaral, 1998). This more complex circuitry model suggests that the DG and CA3 can receive similar information relative to the EC (Johnston and Amaral, 1998). Moreover, areas CA3 and CA1 may communicate with each other via Schaffer collaterals before transmitting information to the EC and vSUB as well as to various brain regions implicated in context-induced drug-seeking behaviors, including the basolateral amygdala (BLA), dorsomedial prefrontal cortex (dmPFC), orbitofrontal cortex, hypothalamus, and nucleus accumbens (NAc) shell (Kelley and Domesick, 1982; van Groen and Wyss, 1990; Witter, 1993; Johnston and Amaral, 1998; Pitkanen et al., 2000; Naber et al., 2001; Fuchs et al., 2005; Ishikawa and Nakamura, 2006; Fuchs et al., 2008; Lasseter et al., 2009; Marchant et al., 2009). In contrast, the DG may only communicate with area CA3 and, as a result, may have a modulatory role in information processing (Steward and Scoville, 1976; Johnston and Amaral, 1998; Naber et al., 2001).
Because the VHp and DG possess different neuroanatomical inputs and outputs, it is possible that these VH subregions make distinct contributions to drug-induced behaviors, including the reinstatement of drug context-induced cocaine seeking. Specifically, the VHp may be more critical for regulating the memory or motivational aspects of this behavior than the DG because it receives direct inputs from the EC independent of the DG and also projects to mesocortical brain regions implicated in drug context-induced cocaine seeking (van Groen and Wyss, 1990; Pikkarainen et al., 1999; Ishikawa and Nakamura, 2006). To test this hypothesis, the effects of GABAB/GABAA agonist-induced temporary neuronal inactivation of the VHp, DG, or the posterior dorsal hippocampus (pDH; an extra-VH control region) were assessed on cocaine-seeking behavior in a distinct cocaine-paired environmental context. Overall, we hypothesized that functional inactivation of the VHp – but not the DG or pDH – would attenuate drug context-induced cocaine seeking.
2.1 EXPERIMENTAL PROCEDURES
2.2 Animals
Male Sprague-Dawley rats (n = 50), weighing 250–300 g at the start of the experiment, were individually housed in a temperature- and humidity-controlled vivarium on a reversed light-dark cycle. Rats were maintained on 20–25 g of rat chow per day with water available ad libitum. The housing and treatment of the rats followed guidelines outlined in the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Sciences, 1996), and the study protocol was approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
2.3 Food Training
In order to expedite the acquisition of cocaine self-administration, rats were trained to press a lever on a fixed ratio 1 (FR1) schedule of food reinforcement (45 mg pellets; Purina, Richmond, IN, USA) in sound-attenuated operant conditioning chambers (26 × 27 × 27 cm high; Coulbourn Instruments, Allentown, PA, USA) during a 16-h overnight food training session. Each active lever response resulted in delivery of one food pellet only; inactive lever responses had no programmed consequences. During the food training session, stimuli subsequently used for contextual cocaine conditioning were not present. Food pellet dispensers were removed from the chambers after food training.
2.4 Surgery
At least 48-h after food training, rats were fully anesthetized using ketamine hydrochloride and xylazine (66.6 and 1.33 mg/kg, i.p., respectively). Chronic indwelling jugular catheters were constructed in house using bent steel cannulae with a screw-type connector (Plastics One, Roanoke, VA, USA), SILASTIC tubing (Dow Corning, Midland, MI, USA), prolite monofilament mesh (Atrium Medical Corp., Hudson, NH, USA), and cranioplastic cement, as described before (Fuchs et al., 2007). The end of the catheter was inserted into the right jugular vein. The catheter ran subcutaneously and exited the back between the scapulae. Immediately following catheterization, all rats were placed into a stereotaxic instrument (Stoelting, Wood Dale, IL, USA) and bilateral stainless-steel guide cannulae (26 gauge; Plastics One) were aimed dorsal to the VHp (−5.2 mm AP, +/−5.4 mm ML, −5.0 mm DV, relative to bregma), DG (−5.7 mm AP, +/−3.7 mm ML, −3.5 DV), or pDH (−5.2 mm AP, +/−4.7 mm ML, −2.5 DV) using standard stereotaxic procedures. The guide cannulae were secured to the skull using three stainless steel screws and cranioplastic cement. To prevent occlusion, stylets (Plastics One) and Tygon caps were used to seal the guide cannulae and catheter, respectively.
Rats were given 5 days for post-operative recovery before the start of the experiment. To extend catheter patency during the recovery period, the catheters were flushed through once daily for 5 days with 0.1 ml of an antibiotic solution of cefazolin (10.0 mg/ml; Schein Pharmaceuticals, Albuquerque, NM, USA) dissolved in heparinized saline (70 U/ml; Baxter Health Care Corp, Deerfield, IL, USA) followed by 0.1 ml of heparinized saline (70 U/ml). Thereafter, catheters were flushed with 0.1 ml of heparinized saline (10 U/ml) before each self-administration session and with 0.1 ml of the cefazolin solution and 0.1 ml of heparinized saline (70 U/ml) after each session. Catheter patency was evaluated before the first self-administration session, as well as periodically during the experiment, using propofol (1mg/0.1ml, i.v. Eli Abbott Lab, North Chicago, IL, USA), an ultrashort-acting sedative-hypnotic that produces a rapid loss of muscle tone only when administered intravenously.
2.5 Contextual Stimuli
Cocaine self-administration and extinction training sessions were conducted in operant conditioning chambers configured to one of two unique environmental contexts that differed along four sensory modalities. Context 1 consisted of a continuous red house light (0.4 fc brightness) on the wall opposite the levers, an intermittent pure tone (80 dB, 1 kHz, 2 sec on, 2 sec off), a pine-scented air freshener strip (4.5 × 2 cm, Car Freshener Corp, Watertown, NY, USA), and wire mesh flooring (26 × 27 cm). Context 2 consisted of an intermittent white stimulus light above the inactive lever (1.2 fc brightness, 2 sec on, 4 sec off), a continuous pure tone (75 dB, 2.5 kHz), a vanilla-scented air freshener strip (4.5 × 2 cm, Sopus Products, Moorpark, CA, USA), and ceramic tile bisecting the steel bar flooring (19 cm × 27 cm). Rats had no exposure to these contextual stimuli prior to self-administration training. As in our previous studies, these stimuli were presented throughout each session independent of responding (Fuchs et al., 2005; Fuchs et al., 2007; Fuchs et al., 2008).
2.6 Cocaine Self-administration Training
Subjects were randomly assigned to undergo cocaine self-administration training in Context 1 or 2. Training was conducted during daily 2-h sessions, during the rats’ dark cycle. The rats’ indwelling catheters were connected to liquid swivels (Instech, Plymouth Meeting, PA, USA) via polyethylene 20 tubing that was encased in steel spring leashes (Plastics One). The swivels were suspended above the operant conditioning chambers and were connected to infusion pumps (Coulbourn Instruments, Allentown, PA, USA). Data collection and reinforcer delivery were controlled using Graphic State Notation software version 2.102 (Coulbourn). Responses on one (active) lever were reinforced on an FR1 schedule of cocaine reinforcement (cocaine hydrochloride; 0.15 mg/0.05 ml per infusion, duration 2 s, i.v.; NIDA, Research Triangle Park, NC, USA). Responses on the other (inactive) lever were recorded but had no programmed consequences. A 20-s time-out period followed each infusion during which lever responses were recorded, but had no programmed consequences. Training continued until the rats successfully obtained ≥ 10 cocaine infusions per session on at least 10 training days (i.e., acquisition criterion).
2.7 Extinction Training
After meeting the acquisition criterion, rats underwent daily 2-h extinction training sessions in the environmental context (Context 1 or 2) that distinctly differed from the cocaine self-administration training context. Active and inactive lever presses were recorded, but had no programmed consequences. On extinction day 4, rats were acclimated to the intracranial infusion procedure. During the adaptation procedure, rats were held gently by the experimenter and injection cannulae were bilaterally inserted into the rats’ guide cannulae to a depth 1 mm below the tip of the guide cannulae. The injectors were left in place for 4 minutes, but no drug was infused. Immediately following the adaptation procedure, rats were placed into the operant conditioning chamber for an extinction session. Extinction training consisted of a minimum of 7 sessions plus additional extinction training sessions, as needed, until the rats reached the extinction criterion (≤ 25 active lever presses per session for 2 consecutive sessions).
2.8 Reinstatement Testing
After the rats reached the extinction criterion, reinstatement of cocaine-seeking behavior was assessed in the cocaine-paired context or in the extinction context over the course of 2 test sessions. Immediately prior to each test session, rats received bilateral microinfusions of the GABAB+A agonist cocktail baclofen+muscimol (BM; 1.0 and 0.1 mM, respectively; pH ~7.0) or phosphate buffered saline vehicle (VEH) into the VHp, DG, or pDH at a volume of 0.5 μl per hemisphere over 2 min. Injectors were left in the guide cannulae for 1 min before and after the infusion. Muscimol, at doses of 1000 ng/1 μL and 20 ng/1 μL, inhibits glucose utilization in a 1.6-mm radius (Martin, 1991) and electrophysiological activity in a 1-mm radius (Arikan et al., 2002). These estimates probably include tissue that exhibits hypoactivity due to reduced synaptic input from inactivated neurons. In the present study, muscimol was administered at 20 ng/0.5 μL. Hence, the area of neural inactivation was probably smaller due to reduced infusion volume. Similar information about the spread of baclofen hydrobromide is not available, but its spread is likely limited by low lipophilicity (Leisen et al., 2003). Importantly, we have used this dose of BM in the past to demonstrate functional differentiation between the NAc core and shell and between the dorsolateral caudate–putamen and overlying somatosensory cortex (Fuchs et al., 2004; Fuchs et al., 2006). Assignment to treatment groups and the order of testing in the previously cocaine-paired versus extinction context were counterbalanced based on mean active lever responding during the last three days of cocaine self-administration training. Subjects received additional extinction training sessions in the extinction context between test sessions until they re-obtained the extinction criterion (≤ 25 lever presses per session for 2 consecutive days). During each test session active and inactive lever presses were recorded, but had no programmed consequences.
2.9 Locomotor Activity Testing
Because intracranial manipulations may produce motor side effects that affect instrumental behavior, the possible nonspecific effects of BM on general locomotor activity were assessed 48 hours after the last test session. Locomotor activity was measured in a novel Plexiglas chamber (42 × 20 × 20 cm) equipped with an array of eight photodetectors and corresponding light sources. Immediately prior to testing, rats received BM or VEH microinfusions into the VHp, DG, or pDH as during reinstatement testing, using the infusion procedures described above (2.8). A computerized activity system (San Diego Instruments, San Diego, CA) recorded the number of consecutive photobeams interrupted by rats moving in the activity chamber during a 2-h test session.
2.10 Histology
After the last experimental session, rats with patent catheters were fully anaesthetized using ketamine hydrochloride and xylazine (66.6 and 1.3 mg/kg, respectively, i.v.), while rats without patent catheters were anesthetized using ketamine hydrochloride and xylazine (199.8 and 3.9 mg/kg, i.p., respectively). Rats were then transcardially perfused using 1×-phosphate-buffered saline (Fisher Scientific) and 10% formaldehyde solution (Sigma). The brains were dissected out and stored in 10% formaldehyde solution prior to being sectioned in the coronal plane at a thickness of 75 μm using a vibratome. The sections were mounted onto gelatin-coated slides and stained using cresyl violet (Kodak, Rochester, NY, USA). Cannula placements were verified using light microscopy. The most ventral portion of each cannula tract was mapped onto schematics of appropriate plates from the rat brain atlas (Paxinos and Watson, 1997).
2.11 Data Analysis
Only data from rats with correct cannula placements were included in the data analysis. To assess potential pre-existing differences between the treatment groups, mixed factorial ANOVAs were used to analyze mean active and inactive lever responses and cocaine intake during the last three days of self-administration training and lever responding during extinction training with cannula placement (VHp, DG, and pDH) and subsequent treatment (BM, VEH) as between-subjects factors and time (day) as the within-subjects factor, where appropriate. To assess the effect of BM and VEH infusions on lever responding during the test sessions, mixed-factorial ANOVAs were used to analyze lever responses with treatment (BM, VEH) as the between-subjects factor and context (cocaine context, extinction context) as the within-subjects factor. To assess the effects of BM on locomotor activity, the number of photobeam breaks were analyzed using mixed-factorial ANOVAs with treatment (BM, VEH) as the between-subjects factor and time (20-minute intervals) as the within-subjects factor, where appropriate. Significant main and interaction effects were further investigated using simple main effects tests or Tukey HSD post hoc tests. Alpha was set at 0.05.
3.1 RESULTS
3.2 Histology
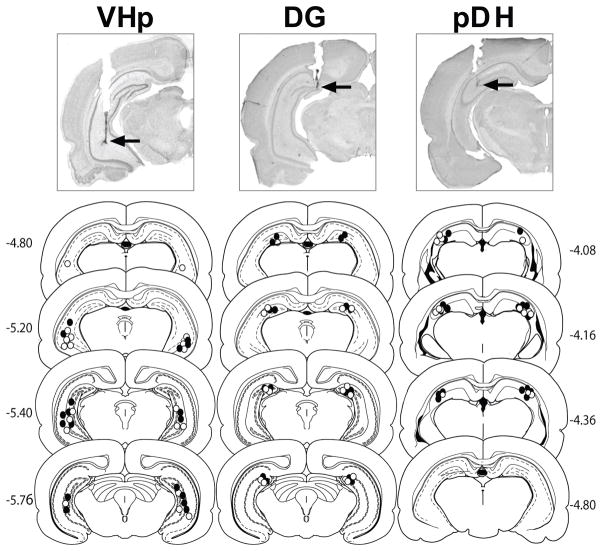
Photomicrographs of representative cannula placements as well as schematic diagrams of the distribution of cannula placements are depicted in Fig 1. The target brain regions were defined as follows: VHp, areas CA3/CA1 of the ventral hippocampus proper; DG, dentate gyrus; and pDH, posterior dorsal hippocampus. For the VHp group, all cannula tips were located within area CA3 or CA1, but area CA2 was within the ~0.5 mm radius of expected drug spread (Martin, 1991; Arikan et al., 2002). The data of rats with misplaced cannulae were excluded from data analysis. High power microscopy confirmed there was no evidence of abnormal tissue damage (i.e., extensive cell loss or gliosis) at the infusion sites. The most ventral points of the cannula tracts were bilaterally located within the target brain regions for the following number of rats: VHp (VEH: n = 10, BM: n = 9); DG (VEH: n = 8, BM: n = 8); pDH (VEH: n = 7, BM: n = 8).
Fig 1.
Schematic and photographic representation of injection cannula placements within the ventral hippocampus proper (VHp), dentate gyrus (DG), or posterior dorsal hippocampus (pDH). The arrows on the photomicrographs identify the most ventral point of the infusion cannula tracts on representative cresyl violet-stained sections. The symbols on the schematics (Paxinos and Watson, 1997) represent the most ventral point of the infusion cannula tracts for rats that received bilateral infusions of baclofen plus muscimol (BM, closed circles) or vehicle (VEH, open circles) into the VHp, DG, or pDH. Numbers indicate the distance from bregma in millimeters.
3.3 Self-administration
Groups with cannulae aimed at the VHp, DG, and pDH exhibited stable active lever responding for cocaine reinforcement during the last three days of self-administration training (day X cannula placement interaction effect, F(4,94) = 0.72, p = 0.58; day main effect, F(2,94) = 0.67, p = 0.52), with a within-subjects variability of <10% in daily cocaine intake. The VHp-cannulated group made fewer active lever responses relative to the DG- and pDH-cannulated groups during the last three days of self-administration training (placement main effect, F(1, 47) = 4.97, p = 0.01, active lever responses: VHp < DG and pDH, Tukey p < 0.05). However, cocaine intake was stable across the last three days of self-administration training for all groups with no differences observed between the VHp-, DG-, and pDH-cannulated groups in the mean number of self-administered cocaine infusions (24.65 ± 1.14 infusions; day X cannula placement interaction effect, F(4,94) = 0.30, p = 0.88; day main effect, F(2,94) = 1.27, p = 0.29; cannula placement main effect, F(1, 47) = 1.75, p = 0.18). Collapsed across groups, the mean daily cocaine intake ± SEM (based on body weight) was approximately 12.33 ± 0.57 mg/kg. Similarly, there were no differences between the VHp-, DG-, and pDH-cannulated groups in inactive lever responding during the last three days of self-administration training (day X cannula placement interaction effect, F(4.94) = 0.19, p = 0.94; day main effect, F(2,94) = 2.88, p = 0.06; cannula placement main effect, F(1,47) = 0.60, p = 0.56).
Separate ANOVAs further indicated that there were no pre-existing differences between the VHp-, DG-, and pDH-cannulated groups that were subsequently assigned to the BM or VEH treatment condition in active lever responding (Fig 2A–C; all subsequent treatment and day main and interaction effects: F(1–2, 14–34) = 0.24–1.21, p = 0.32–0.63) or in cocaine intake (all subsequent treatment and day main and interaction effects: F(1–2, 14–34) = 0.01–1.12, p = 0.34–0.95) during the last three days of self-administration training. In the DG-cannulated rats, the BM treatment group made significantly fewer inactive lever responses relative to the VEH treatment group during the last three days of self-administration (subsequent treatment main effect, F(1, 14) = 6.92, p = 0.02), but there were no differences between groups in the pattern of inactive lever responses across self-administration training days (Fig. 2E; subsequent treatment X day main effect, (F(2, 28) = 0.87, p = 0.43; day main effect, F(2, 28) = 1.00, p = 0.38). Similarly, there were no pre-existing differences between the VHp- and pDH-cannulated groups that were subsequently assigned to the BM or VEH treatment condition in inactive lever responding (Fig 2D, 2F; all subsequent treatment and day and interaction effects: F(1–2, 14–34) = 0.24–1.35, p = 0.27–1.24).
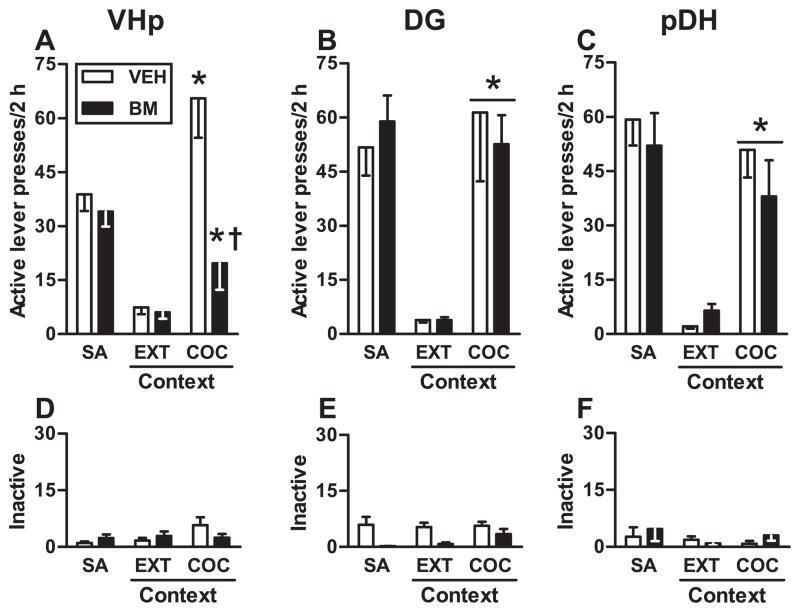
Fig 2.
Functional inactivation of the VHp – but not the DG or pDH – attenuates drug context-induced reinstatement of cocaine-seeking behavior. The panels depict non-reinforced active and inactive lever responses (mean/2h ± SEM) during testing in the extinction context (EXT context) and the previously cocaine-paired context (COC context). Immediately before testing, BM or VEH infusions were administered into the VHp (A,D), DG (B,E), or pDH (C,F). Asterisks represent significant difference relative to responding in the extinction context (A: ANOVA context simple main effect, Tukey test, p < 0.01; B, C: ANOVA context main effect, p < 0.05). Dagger represents significant difference relative to VEH treatment (A: ANOVA treatment simple main effect, Tukey test, p < 0.01).
3.4 Extinction
Upon removal of cocaine reinforcement during extinction training, active and inactive lever responding in the VHp-, DG-, and pDH-cannulated groups gradually declined (active lever: day main effect, F(6, 282) = 33.25, p < 0.0001; day 1 > day 2–7, Tukey test, p < 0.05; inactive lever: day main effect, F(6, 282) = 14.070, p < 0.0001; day 1 > day 2–7, Tukey test, p < 0.05), and there were no differences between the groups in active and inactive lever responding during the first seven days of extinction training (all cannula placement main and interaction effects: F(1–12, 6–282) = 0.082–0.56, p = 0.55–1.00). There were also no pre-existing differences in active or inactive lever responding during extinction training between the VHp-, DG-, and pDH-cannulated groups that were subsequently assigned to the BM or VEH treatment condition (all subsequent treatment main and treatment X day interaction effects: F(1–6, 17–102) = 0.05–1.33, p = 0.251–0.85). Furthermore, separate analyses revealed that these groups required the same mean number of days (± SEM) to reach the extinction criterion (t(13–17) = 0.08–1.00, p = 0.33–0.94; VEH mean = 7.00–7.11 ± 0.00–0.11, BM mean = 7.00–7.75 ± 0.00–0.75).
3.5 VHp Functional Inactivation Attenuates Drug Context-induced Reinstatement of Cocaine-seeking Behavior
During the reinstatement test session, exposure to the previously cocaine-paired context increased active lever responding in the VHp-cannulated group relative to responding in the extinction context (Fig. 2A; context main effect, F(1,17) = 29.20, p < 0.001). However, BM pretreatment administered into the VHp altered active lever responding relative to VEH pretreatment in a context-specific manner (treatment X context interaction effect, F(1,17) = 11.17, p = 0.004; treatment main effect, F(1,17) = 11.08, p = 0.004). Specifically, BM pretreatment administered into the VHp attenuated active lever responding in the cocaine-paired context (Tukey test, p < 0.01) but not in the extinction context relative to VEH pretreatment. In contrast, exposure to the cocaine-paired context failed to alter inactive lever responding relative to responding in the extinction context (Fig. 2D; context main effect, F(1,17) = 2.79, p = 0.11). Furthermore, BM pretreatment administered into VHp failed to alter inactive lever responding in either context relative to VEH pretreatment (treatment X context interaction effect, F(1,17) = 1.80, p = 0.20, treatment main effect, F(1,17) = 0.63, p = 0.44)
3.6 Effects of DG Functional Inactivation on Drug Context-induced Reinstatement of Cocaine-seeking Behavior
Exposure to the cocaine-paired context elicited robust active lever responding in the DG-cannulated group relative to responding in the extinction context (Fig. 2B; context main effect, F(1,14) = 25.46, p < 0.0001). BM pretreatment administered into the DG failed to alter active lever responding in either context relative to VEH pretreatment (treatment X context interaction effect, F(1,14) = 0.09, p = 0.77; treatment main effect, F(1,14) = 0.31, p = 0.59). Exposure to the cocaine-paired context did not alter inactive lever responding relative to responding in the extinction context (Fig. 2E; context main effect, F(1,14) = 4.52, p = 0.052). Furthermore, BM pretreatment did not alter inactive lever responding in either context relative to VEH pretreatment (treatment X context interaction effect, F(1,14) = 0.09, p = 0.68; treatment main effect, F(1,14) = 0.18, p = 0.09).
3.7 Effects of pDH Functional Inactivation on Drug Context-induced Reinstatement of Cocaine-seeking Behavior
Exposure to the cocaine-paired context potentiated active lever responding in the pDH-cannulated groups relative to responding in the extinction context (Fig. 2C; context main effect, F(1,13) = 40.71, p < 0.001). BM pretreatment administered into the pDH failed to alter active lever responding in either context relative to VEH pretreatment (treatment X context interaction effect, F(1,13) = 1.88, p = 0.194; treatment main effect, F(1,13) = 0.39, p = 0.54). Exposure to the cocaine-paired context did not alter inactive lever responding relative to responding in the extinction context (Fig. 2F; context main effect, F(1,13) = 0.40, p = 0.58). Furthermore, BM pretreatment administered into the pDH did not alter inactive lever responding in either context relative to VEH pretreatment (treatment X context interaction effect, F(1,13) = 3.10, p = 0.10, treatment main effect, F(1,13) = 0.35, p = 0.57).
3.8 Locomotor Activity
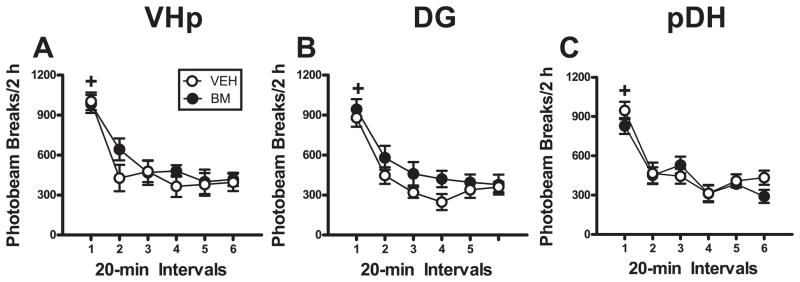
Separate ANOVAs revealed that BM pretreatment administered into the VHp, DG, or pDH did not alter locomotor activity relative to VEH pretreatment (Fig. 3). In all groups, the number of photobeam breaks declined at a similar rate over the six 20-min intervals of the locomotor test session as the groups habituated to the novel context (all time main effects, F(5, 65–85) = 22.24–113.25, p < 0.0001; interval 1 > intervals 2–6; Tukey test, p < 0.01). In addition, BM pretreatment administered into the VHp, DG, or pDH did not alter the number of photobeam breaks relative to VEH pretreatment (all treatment main and interaction effects, F(1–5, 65–85) = 0.09–2.45, p = 0.14–0.76).
Fig 3.
Functional inactivation of the VHp, DH, or pDH failed to alter locomotor activity measured as the number of photobeam breaks triggered by the movement of subjects in a novel context. The panels depict horizontal photobeam breaks (mean/2h ± SEM) during testing in a novel context immediately after BM or VEH infusions were administered into the VHp (A), DG (B), or pDH (C). Plus signs represent significant difference relative to all other time points (ANOVA time simple main effect, Tukey test, p < 0.05).
4.1 DISCUSSION
The findings in the present study demonstrate that the reinstatement of drug context-induced cocaine-seeking behavior critically relies on the functional integrity of the VH. Interestingly, the VH makes a subregion-specific contribution to drug context-induced cocaine seeking such that neural processing in the VHp – but not the DG – is necessary for the expression of this behavior. Specifically, functional inactivation of the VHp disrupted the ability of a drug-paired context to reinstate cocaine-seeking behavior (Fig. 2A), whereas functional inactivation of the DG failed to alter this behavior (Fig. 2B). BM pretreatment administered into the VHp did not alter either active lever responding in the extinction context (Fig. 2A), inactive lever responding in the drug-paired context (Fig. 2D), or locomotor behavior in a novel context (Fig. 3A). Similarly, previous studies have shown that functional inactivation of the VHp does not decrease instrumental responding for cocaine (Atkins et al., 2008) or food reinforcement (Rogers and See, 2007) and fails to alter extinction learning or reinstatement responding following a saline-priming injection (Rogers and See, 2007). While the specific contribution of the VHp to drug context-induced cocaine seeking is difficult to ascertain given the inherent complexity underlying motivated behaviors, together these results indicate that functional inactivation of the VHp impaired either the expression of drug context-induced motivation for cocaine or the memory of cocaine-context associations rather than causing non-specific deficits in instrumental or motor performance.
Importantly, results expand upon previous studies indicating that the VH is involved in associative learning and memory processes that underlie aversive conditioned behaviors. For instance, VH lesions and functional inactivation impair the acquisition and expression of context-specific fear memories (Maren, 1999; Richmond et al., 1999; Zhang et al., 2001; Yoon and Otto, 2007). Moreover, the current findings complement evidence that the functional integrity of the VH is necessary for both cue-induced and cocaine-primed cocaine seeking (Sun and Rebec, 2003; Rogers and See, 2007). Hence, the VH appears to make a fundamental contribution to cocaine-seeking behavior independent of the mode of reinstatement.
Remarkably, functional inactivation of the pDH failed to alter context-induced cocaine seeking (Fig. 1C) even though functional inactivation of the anterior DH disrupts this behavior (Fuchs et al., 2005; Fuchs et al., 2007). Anatomical studies have demonstrated that the hippocampus receives distinct, topographically-organized inputs from the entorhinal cortex along its anterior-posterior axis and that the corresponding hippocampal domains have different patterns of neural connectivity (Burwell, 2001; Cenquizca and Swanson, 2007; Dong et al., 2009; Fanselow and Dong, 2010). These unique anatomical connections may contribute to functional differences between the anterior and posterior regions of the DH. Thus, while the microcircuits have yet to be identified, our findings indicate that the contributions of both the VH and DH are subregion-specific with respect to drug context-induced cocaine-seeking behavior.
No studies to date have investigated the functional heterogeneity of the VH with respect to its involvement in drug context-induced cocaine seeking. However, evidence does suggest that areas CA3 and CA1, which comprise the VHp, play a critical role in context-mediated learning and spatial memory, while the DG may play a more circumscribed role in preparing spatial representations for the CA3/CA1 network rather than in storing or retrieving these representations (Jung and McNaughton, 1993; Chaillan et al., 1999; Rolls and Kesner, 2006). For instance, area CA1 – but not the DG – exhibits enhanced neural activation during the acquisition of contextual fear conditioning and the retrieval of context-fear associations, while pretraining lesions of area CA3 or CA1 prevent contextual fear conditioning and impair performance on a water maze task (Neisewander et al., 2000; Kudo et al., 2004). In accordance with this, NMDA receptor knockdown in area CA3 impairs the retrieval of contextual memories in the Morris water maze task and this is associated with decreased neural activation within area CA1 (Stubley-Weatherly et al., 1996; Nakazawa et al., 2002; Hunsaker and Kesner, 2008). Interestingly, however, exposure to cocaine-paired contextual stimuli enhances Fos protein expression in both area CA1 and the DG concomitant with cocaine-seeking behavior (Neisewander et al., 2000). However, in that study, drug context-induced cocaine seeking and Fos protein expression were assessed following an experimenter-imposed drug-free period (i.e. abstinence), while in the present study, the reinstatement of cocaine seeking was assessed following explicit extinction training. Thus, results from the present study further this line of research by suggesting that neural activity in the DG may not be necessary for the performance of drug context-induced cocaine-seeking behavior despite Fos protein expression in this brain region or that the recruitment of the DG may be inhibited by extinction training.
Differences between the neuroanatomical connections of the VHp and the DG may underlie their distinct contributions to drug context-induced cocaine seeking. In contrast to previous models of information processing that posited the DG as the primary point of entry for information into the VH, pyramidal cells in areas CA3 and CA1 are innervated by the EC, which indicates that information intended for the VH can bypass the DG (Steward and Scoville, 1976; Mizumori et al., 1989; Ishizuka et al., 1990; Witter, 1993; Johnston and Amaral, 1998). In fact, the EC sends more numerous projections to area CA3 than to the DG (Rolls and Kesner, 2006), and neural activation in area CA1 can occur despite loss of input from the DG (Mizumori et al., 1989). Thus, the VHp may be anatomically well-positioned to receive and process inputs from the EC and then transmit this information to other brain regions implicated in drug context-induced cocaine-seeking, including the BLA, dmPFC, orbitofrontal cortex, hypothalamus, and NAc shell (Kelley and Domesick, 1982; van Groen and Wyss, 1990; Witter, 1993; Johnston and Amaral, 1998; Pitkanen et al., 2000; Naber et al., 2001; Fuchs et al., 2005; Ishikawa and Nakamura, 2006; Fuchs et al., 2008; Lasseter et al., 2009; Marchant et al., 2009). It should be noted that the VHp also contains a narrow band of cells between areas CA3 and CA1 that comprise the CA2 field. However, area CA2 primarily shares collaterals with area CA3, indicating it is mainly involved in intra-hippocampal information processing (Ishizuka et al., 1990), and no known studies to date have explored the independent contribution of area CA2 to behavior.
Future research is necessary to identify the specific mesocorticolimbic brain regions with which the VHp interacts to regulate drug-induced behavior. The BLA may be a good candidate given that the VH and the BLA share dense reciprocal connections and electrical stimulation of area CA1 is sufficient to elicit electrophysiological activity in the BLA (Pitkanen et al., 2000; Fuchs et al., 2005; Ishikawa and Nakamura, 2006). In fact, previous work from our lab has established that serial information processing by the BLA and DH is necessary for the expression of drug context-induced cocaine seeking despite comparatively sparser anatomical connections between these structures than those between the BLA and VH (Pikkarainen et al., 1999; Fuchs et al., 2007). Furthermore, the VH, DH, and BLA project to the dmPFC, a brain region theorized to initiate various forms of cocaine-seeking behavior via its projections to the NAc core (Fuchs et al., 2005). In addition, the VHp interacts with subcortical brain regions indirectly via communication with the vSUB, the primary output structure for the VH. Because the vSUB has been shown to play a critical role in the acquisition of context-cocaine associations that subsequently mediate drug context-induced cocaine seeking (Atkins et al., 2008), it is possible that functionally significant interactions between the VH and vSUB are necessary for the expression of this behavior. Alternatively, the VHp may mediate drug context-induced cocaine-seeking behaviors via direct communication with the NAc shell, another brain region that is critical for the expression of context-induced drug seeking (Bossert et al., 2006; Bossert et al., 2007; Fuchs et al., 2008). VH glutamatergic projections converge with VTA dopaminergic input on neurons within the NAc shell such that VH stimulation enhances NAc shell dopaminergic neurotransmission (Sesack and Pickel, 1990; Johnson et al., 1994). Communication between the VH and NAc shell may indeed facilitate context-induced cocaine seeking given that inhibiting either glutamatergic neurotransmission or D1 receptor stimulation in the NAc shell prevents context-induced heroin seeking (Bossert et al., 2006; Bossert et al., 2007; Fuchs et al., 2008). In future studies, it will be important to systematically identify the neural subcircuits via which the VHp regulates drug context-induced cocaine seeking as well as to determine the neuropharmacological mechanisms within the VHp that mediate drug-induced behaviors. Given the strong contextual control over addictive behavior, understanding the neural mechanisms by which the VH contributes to drug context-induced cocaine seeking will be important for developing effective pharmacotherapies for the treatment of cocaine addiction.
Acknowledgments
The authors would like to thank Kate Cowhey, John Tobben, Stephanie Traina, Audrey Wells, Portia West, and Amy Zipursky for excellent technical assistance and insightful comments on an earlier version of this manuscript. This work was supported by National Institute on Drug Abuse (NIDA) R01 DA017673, a NIDA R01 grant supplement to promote diversity in health-related research (DA017673-S1), NIDA T32 DA07244, the University of North Carolina at Chapel Hill Junior Faculty Development Award, and the Mason and Linda Stephenson Faculty Award.
List of Abbreviations
- BLA
basolateral amygdala
- BM
baclofen + muscimol
- DG
dentate gyrus
- DH
dorsal hippocampus
- dmPFC
dorsal medial prefrontal cortex
- EC
entorhinal cortex
- GABA
gamma-aminobutyric acid
- NAc
nucleus accumbens
- OFC
orbitofrontal cortex
- pDH
posterior dorsal hippocampus
- VEH
vehicle
- VH
ventral hippocampus
- VHp
ventral hippocampus proper
- vSUB
ventral subiculum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arikan R, Blake NM, Erinjeri JP, Woolsey TA, Giraud L, Highstein SM. A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. J Neurosci Methods. 2002;118:51–57. doi: 10.1016/s0165-0270(02)00143-7. [DOI] [PubMed] [Google Scholar]
- Atkins AL, Mashhoon Y, Kantak KM. Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2008;90:481–491. doi: 10.1016/j.pbb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Hippocampal memory system function and the regulation of cocaine self-administration behavior in rats. Behav Brain Res. 2004;151:225–238. doi: 10.1016/j.bbr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD. Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. J Comp Neurol. 2001;437:17–41. doi: 10.1002/cne.1267. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillan FA, Truchet B, Roman FS, Soumireu-Mourat B. Early polysynaptic potentiation recorded in the dentate gyrus during an associative learning task. Neuroscience. 1999;94:443–451. doi: 10.1016/s0306-4522(99)00304-8. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW, Chen L, Fanselow MS, Toga AW. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci U S A. 2009;106:11794–11799. doi: 10.1073/pnas.0812608106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, McDonald RJ. Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus. 2001;11:187–200. doi: 10.1002/hipo.1036. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem. 2008;89:61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. J Neurophysiol. 2006;96:2134–2138. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Aylward RL, Hussain Z, Totterdell S. Input from the amygdala to the rat nucleus accumbens: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience. 1994;61:851–865. doi: 10.1016/0306-4522(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Johnston D, Amaral DG. In the Synaptic Organization of the Brain. Oxford University Press; New York: 1998. Hippocampus. [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kudo K, Qiao CX, Kanba S, Arita J. A selective increase in phosphorylation of cyclic AMP response element-binding protein in hippocampal CA1 region of male, but not female, rats following contextual fear and passive avoidance conditioning. Brain Res. 2004;1024:233–243. doi: 10.1016/j.brainres.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:1370–1381. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisen C, Langguth P, Herbert B, Dressler C, Koggel A, Spahn-Langguth H. Lipophilicities of baclofen ester prodrugs correlate with affinities to the ATP-dependent efflux pump P-glycoprotein: relevance for their permeation across the blood-brain barrier? Pharm Res. 2003;20:772–778. doi: 10.1023/a:1023437603555. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29:1331–1342. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999;113:283–290. doi: 10.1037//0735-7044.113.2.283. [DOI] [PubMed] [Google Scholar]
- Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127:160–164. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Barnes CA, McNaughton BL. Reversible inactivation of the medial septum: selective effects on the spontaneous unit activity of different hippocampal cell types. Brain Res. 1989;500:99–106. doi: 10.1016/0006-8993(89)90303-x. [DOI] [PubMed] [Google Scholar]
- Naber PA, Lopes da Silva FH, Witter MP. Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus. 2001;11:99–104. doi: 10.1002/hipo.1028. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain Stereotaxic Coordinates. Academic Press; New York: 1997. [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87:688–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: theoretical and treatment implications. Int J Addict. 1990;25:957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug Alcohol Depend. 2000;60:207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res. 1990;527:266–279. doi: 10.1016/0006-8993(90)91146-8. [DOI] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Stubley-Weatherly L, Harding JW, Wright JW. Effects of discrete kainic acid-induced hippocampal lesions on spatial and contextual learning and memory in rats. Brain Res. 1996;716:29–38. doi: 10.1016/0006-8993(95)01589-2. [DOI] [PubMed] [Google Scholar]
- Sun WL, Rebec GU. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taepavarapruk P, Phillips AG. Neurochemical correlates of relapse to d-amphetamine self-administration by rats induced by stimulation of the ventral subiculum. Psychopharmacology (Berl) 2003;168:99–108. doi: 10.1007/s00213-002-1337-2. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol. 1990;302:515–528. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Witter MP. Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus. 1993;3(Spec No):33–44. [PubMed] [Google Scholar]
- Yoon T, Otto T. Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiol Learn Mem. 2007;87:464–475. doi: 10.1016/j.nlm.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav Brain Res. 2001;126:159–174. doi: 10.1016/s0166-4328(01)00256-x. [DOI] [PubMed] [Google Scholar]