Abstract
Objective
Evaluation of single nucleotide polymorphisms (SNPs) in the interleukin-10 promoter (−592 and −819) on risk for death after burn injury.
Methods
Association between the IL-10 SNPs and outcome after burn injury was evaluated in a cohort of 265 patients from Parkland Hospital, Dallas, TX with ≥15% TBSA burns without non-burn trauma (ISS ≤ 16), traumatic or anoxic brain injury or spinal cord injury, who survived >48 h under an IRB-approved protocol. Clinical data were collected prospectively and genotyping was conducted by TaqMan assay. Whole blood from 31 healthy volunteers was stimulated with LPS (100 ng/mL) to determine the level of IL-10 expression for each allele by enzyme-linked immunosorbent assay (ELISA).
Results
After adjustment for percent total body surface area (TBSA) burned, inhalation injury, age, gender, and race/ethnicity, carriage of −592A and/or −819T was significantly associated (P = 0.014) with a decreased risk for death (adjusted odds ratio: 0.404; 95% CI: 0.197–0.829). As the candidate SNPs were in complete linkage disequilibrium, it was not possible to distinguish which allele was associated with decreased mortality risk. Age, inhalation injury, and full-thickness burn size were significantly associated with increased risk for death. In the LPS stimulated blood of healthy controls, carriage of the −592A and/or −819T allele demonstrated a trend for decreased levels of IL-10 (P = 0.079).
Conclusion
Carriage of the −592A and/or −819T allele in the IL-10 promoter appears to reduce the risk for death after burn injury.
Keywords: interleukin-10, genetic association, burn injury, mortality
INTRODUCTION
Burn injuries remain a significant threat to individuals with approximately one million burn injuries occurring annually within the United States [1]. Additionally, the third leading cause of fatal home injuries is the result of fire or burn injury [2]. While significant advances in burn wound care, combined with early, aggressive fluid resuscitation and wound excision have led to a decrease in mortality across all age groups, burn-related morbidity remains high [3, 4].
Despite intensive investigation and increased understanding of the pathophysiology of the post-burn clinical course, the identification of those patients who are at increased risk for infectious complications or mortality after burn injury remains extremely difficult. Genetic predisposition may explain a portion of this individual variation in outcome. Indeed, significant associations have been observed between single nucleotide polymorphisms (SNPs) and clinical outcome after traumatic injury [5–8].
One of the cytokines that provides negative feedback control to the pro-inflammatory response to burn injury is interleukin-10 (IL-10). This anti-inflammatory cytokine is known to inhibit the production of proinflammatory cytokines (IL-1β, IL-6, IL-12, TNF-α) and chemokines. Although IL-10 is primarily produced by TH2 cells, it can be produced by other cells crucial to the inflammatory response [9].
Associations have been reported between carriage of polymorphisms within the IL-10 promoter and increased risk for Crohn's disease [10], rheumatoid arthritis [11], oral cancer [12], and Alzheimer's disease [13]. In addition, a study of critically ill patients observed a significant association between promoter polymorphisms at position −592 (rs# 1800872) and −819 (rs#1800871) for IL-10 with increased risk for death and decreased levels of circulating IL-10 [14]. However, the literature contains conflicting reports regarding the functional effects of these IL-10 promoter polymorphisms upon levels of IL-10 in the blood [9, 14].
Given the anti-inflammatory effects of IL-10 and previously published genetic associations for increased risk for several diseases, we chose to study associations between IL-10 promoter polymorphisms −592 and −819 with outcome following severe burn injury.
MATERIALS AND METHODS
Patients
Patients admitted to the burn intensive care unit (BICU) unit at Parkland Memorial Hospital (Dallas, TX) with either ≥15% total body surface area (TBSA) burns or with third degree burns covering ≥5% TBSA were prospectively enrolled in this study to evaluate the relationship between IL-10 promoter genotype and risk for post-burn complications. Under an IRB-approved protocol, DNA was isolated from residual blood from standard care blood draws. In order to remove confounding variables unrelated to burn injury, individuals were excluded if they presented with significant non-burn-related trauma (injury severity score (ISS) ≥ 16), traumatic or anoxic brain injury, spinal cord injury, were HIV/AIDS positive, had active malignancy, or failed to survive more than 48 h post-admission. Clinical data were recorded into a computerized database; once clinical data collection was complete, all patient identifiers were removed. Unique anonymous study identifiers linked clinical data and genotyping results.
Methods
Genomic DNA was extracted using a Puregene DNA Purification Kit (Minneapolis, MN) from unfractionated whole blood, per manufacturer's recommendations, and stored at −20°C awaiting amplification by polymerase chain reaction (PCR). TaqMan genotyping assays were obtained from Applied Biosystems, Inc. (Foster City, CA). The assay identification code for each respective SNP is C___1747362_10 (rs1800871) and C___1747363_10 (rs1800872). Genotypes were determined by real-time PCR using the commercially available TaqMan probes (Applied Biosystems, Inc.). All amplifications were carried out in an ABI 7900HT thermal cycler (Applied Biosystems, Inc.) using TaqMan Genotyping Master Mix and following the manufacturer's recommended amplification conditions.
Assay for LPS-Stimulated Leukocyte IL-10 Production
Whole blood from the 31 healthy volunteers was collected into EDTA Vacutainer tubes. Assays were performed after diluting the blood samples 9:1 in RPMI-1640 media supplemented with glutamine and antibiotics. Aliquots of 2 mL of diluted blood per well were equally divided into LPS stimulated (100 ng/mL of E. coli LCD25; List Biological Laboratories, Campbell, CA) and non-LPS groups in 24-well plates (Corning Inc., Corning, NY). Each experimental condition was conducted in triplicate. Plates were incubated in 5% CO2 for 24 h at37°C and supernatants were harvested and stored at −80°C until assayed for IL-10. A single aliquot of each supernatant was thawed and used to measure IL-10 level by enzyme-linked immunosorbent assay (ELISA) (Biosource, Carlsbad, CA) following the manufacturer's recommended protocol. All ELISA measurements were made using undiluted samples of cell culture supernatants.
Categorical data were compared using χ2 and continuous data were compared by the Mann-Whitney U test. Multivariate logistic regression was used to simultaneously evaluate the effects of multiple genetic, demographic, and injury characteristics upon risk for post-burn mortality. A P value of ≤ 0.05 was used to infer statistical significance. Descriptive statistics included counts and percentages for categorical variables and medians with associated 25th and 75th quartiles for continuous data. Adjusted odds ratios (aORs) obtained from the regression analysis were presented with their associated 95% confidence intervals. Actual P values were reported for all analyses. Statistical Package for the Social Sciences (SPSS, Inc. Chicago, IL) was used for calculations.
RESULTS
A total of 265 patients meeting eligibility criteria were enrolled in this study. Table 1 presents demographics, injury statistics, and clinical outcome for these patients. The median age of the cohort was 35 y (range <1–87 y) and the majority of the cohort was male (75%). Overall mortality was 15%, with 50 patients (19%) developing complicated sepsis, according to ABA consensus criteria [15]. Mortality in the cohort was stratified on IL-10 genotype (−592/−819) as follows, 23 (24.5%) CC/CC, 14 (13%) CA/CT, and three (13%) AA/TT (Fig. 1). Based on self-reported ethnicity, the patient cohort was primarily Caucasian (56%) and Hispanic (24%), with a significant number of African Americans (17%). Nine individuals (3%) were categorized as `other'. The median percentage TBSA burned was 30% with a median full thickness burn size of 15% TBSA. When patients were stratified based upon carriage of the −592A/−819T allele, age (P = 0.357), gender (P = 0.308), burn size (P = 0.910), and inhalation injury (P = 0.355) did not significantly differ between groups. Genotype frequencies were found to be in Hardy-Weinberg equilibrium, with the exception that, as previously reported, the −592A and −819T alleles were in complete linkage disequilibrium [14].
TABLE 1.
Demographic and Burn Injury Characteristics for the Entire Cohort of 265 Patients. Data are Presented for the Overall Cohort and Stratified by Development of Complicated Sepsis
| Variable (n = 265) | Overall (n=265) Median (IQR); Count (percentage) | No Sepsis (n=215) Median (IQR); Count (percentage) | Complicated Sepsis (n=50) Median (IQR); Count (percentage) | Nominal P value |
|---|---|---|---|---|
| Age (y) | 35 (22–51) | 35 (22–50) | 39 (23–53) | 0.649 |
| Male gender | 198 (75%) | 163 (76%) | 35 (70%) | 0.394 |
| Race/ethnicity | 0.134 | |||
| Caucasian | 148 (56%) | 122 (57%) | 26 (52%) | |
| Hispanic | 63 (24%) | 52 (24%) | 11 (22%) | |
| African-American | 45 (17%) | 35 (16%) | 10 (20%) | |
| Other | 9 (3%) | 6 (3%) | 3 (6%) | |
| Burn injury | ||||
| TBSA | 30 (20–45) | 26 (20–39) | 50 (30–75) | 0.001* |
| Full thickness TBSA | 15 (8–34) | 13 (6–25) | 42 (19–66) | <0.001† |
| Inhalation | 50 (19%) | 38 (18%) | 12 (24%) | 0.303 |
| Outcome | ||||
| Mortality | 40 (15%) | 20 (9%) | 20 (40%) | <0.001† |
| IL-10 Genotype | ||||
| −592 CC/−819 CC | 117 (44%) | 95 (44%) | 22 (44%) | 0.879 |
| −592 CA/−819 CT | 122 (46%) | 98 (46%) | 24 (48%) | |
| −592 AA/−819 TT | 26 (10%) | 22 (10%) | 4 (8%) |
IQR = inter quartile range.
Significant at P < 0.05.
Significantat P < 0.01.
FIG. 1.
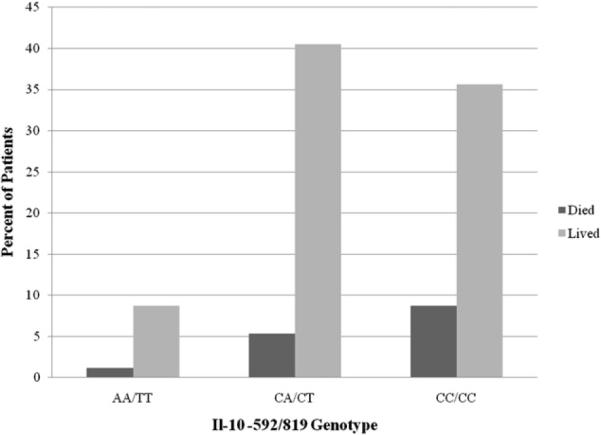
Mortality risk of burn patients stratified on IL-10−592/819 genotype.
Using multivariate logistic regression, we explored the influence of multiple variables, including age, percent TBSA, presence of inhalation injury, gender, race/ethnicity, and IL-10 promoter genotype on the risk for death. Carriage of IL-10 promoter allele −819T (aOR = 0.404; 95% CI 0.197–0.829; P = 0.014) was associated with a significantly lower risk for mortality. Conversely, age (aOR = 1.091; 95% CI 1.058–1.125; P < 0.001), TBSA (aOR = 1.069; 95% CI 1.043–1.096; P < 0.001), and inhalation injury (aOR = 4.348; 95% CI 1.692–11.172; P = 0.002), were significantly associated with an increased risk for mortality following burn injury (Table 2). When the effect of the SNP was examined in a receiver operator curve, addition of the SNP did not significantly affect the area under the curve (0.874) compared with the curve for burn size and age (0.876). In separate multivariate logistic regression analyses, IL-10 genotype was not significantly associated with risk for complicated sepsis (P = 0.351). Levels of IL-10 within each burn patient sample were not measured. DNA was isolated from residual blood from a standard care blood draw, which had been stored at 4°C for at least 24 h, which precluded accurate measurement of plasma IL-10 levels from the burn patient samples.
TABLE 2.
Odds Ratios for Mortality After Burn Injury Following Adjustment for Multiple Factors, as Determined by Multivariate Logistic Regression. Age and Total Burn Surface Area Were Analyzed as Continuous Variables; Odds Ratios are for Each Additional Year of Age or Each 1% Increase in Burn Size
| 95% CI for odds ratio |
||||
|---|---|---|---|---|
| (n = 265) Variable | Odds ratio | Lower | Upper | P value |
| Age | 1.091 | 1.058 | 1.125 | <0.001 |
| Total burn surface area | 1.069 | 1.043 | 1.096 | <0.001 |
| Gender (female) | 0.960 | 0.383 | 2.406 | 0.930 |
| Inhalation injury | 4.348 | 1.692 | 11.172 | 0.002 |
| Race/ethnicity | 1.405 | 0.896 | 2.204 | 0.138 |
| IL-10-592A/-819T | 0.404 | 0.197 | 0.829 | 0.014 |
IL-10 promoter region (−592/−819) genotypes of the 31 healthy volunteers were 19 (53%) CC/CC, 11 (30%) CA/CT, and 6 (17%) AA/TT. Carriers of the A allele had decreased levels of IL-10 production following 100ng/mL LPS (16.6 ± 13.3 A_/T_ versus 26.7 ± 17.3 CC/CC). However, differences between the groups were not statistically significant (P = 0.079).
DISCUSSION
In our cohort of burn patients, carriage of the −592A/−819T allele in the IL-10 promoter region was significantly associated with decreased risk for mortality. With the common inflammatory cellular response to burn trauma, it is necessary to have biologic feedback mechanisms to regulate the inflammatory response. IL-10 is a known anti-inflammatory cytokine that has the ability to inhibit the production of pro-inflammatory cytokines and chemokines [9]. During response to burn trauma, levels of IL-10 are known to increase significantly in an attempt to regulate the effects of pro-inflammatory response [16]. The candidate SNPs in this study are located within the promoter region for IL-10.
Our finding of an association between IL-10 −592A/−819T and mortality following burn injury is in contradiction to a previous study of mortality in a Scottish cohort of ICU patients. In the study by Lowe and colleagues, a total of 67 consecutively enrolled ICU patients and 132 healthy volunteers were examined for association between IL-10 polymorphisms and sepsis, mortality, and IL-10 production [14]. This study reported an association between the IL-10 −592A/−819T allele, decreased IL-10 levels, and increased risk for mortality. The genetic association with increased mortality is in direct contradiction to the present study, where we found the −592A/−819T polymorphism to be associated with decreased mortality following burn trauma.
Although the effect of the IL-10 592A/−819T allele on mortality is different between our study and Lowe et al., the values of IL-10 production following LPS stimulation in our healthy volunteers was concordant with Lowe et al., albeit non-significant in this study population of healthy control subjects.
Although the divergent results regarding the two studies are perplexing, there are inherent methodological differences between Lowe et al. and the present study. Unlike Lowe and colleagues, who enrolled all comers to a general ICU, we limited our study to burn patients. Therefore, the current study had a more homogenous patient cohort. Biologic response to burn injury elicits a common pro-inflammatory response, of which IL-10 is an important component by providing necessary negative feedback regulation against pro-inflammatory cytokines, whereas the critically ill patients admitted to the ICU in the Lowe et al. study potentially have different etiologies that result in admission to the ICU. Depending on the injury mechanism, decreased or increased levels of IL-10 could be more beneficial. Responses to trauma or critical illness potentially elicit different cellular responses and could be the underlying reason for the apparent contradiction of the present study and Lowe et al. Csontos et al. found a significant difference in IL-10 levels on admission between survivors and non-survivors following severe burn trauma [17]. IL-10 levels in non-survivors was elevated relative to survivors even though there was no significant difference in total burn size between survivors and non-survivors. When considering the effect of IL-10 promoter polymorphisms on IL-10 levels presented by Lowe et al. [14], and supported by the present study, the findings of Csontos et al. [17] provide a potential explanation for the divergent results between Lowe et al. [14] and the present study.
Furthermore, when discussing divergent results between the data presented here and other reports in the literature, it must be admitted that this study suffers from several weaknesses. First, the patient cohort was drawn from a single institution. Replication of these findings in an independent group of burn patients is needed before strong conclusions may be drawn from these data. In addition, this study, and many other tests for association between outcome from trauma and variant alleles, have focused primarily upon individual polymorphisms. The simultaneous evaluation of association between multiple SNPs and outcome has not been extensively examined. In an effort to resolve problems with single gene association studies and conflicting results within the literature, we must move to multi-gene associations that not only examine single gene effects but also epistatic interactions between loci to further explain individual response to thermal injury and trauma.
The mechanism by which the IL-10 polymorphisms may reduce risk for mortality remains unknown. Although the source of the DNA for this study enhanced patient accrual, it precluded analysis of plasma IL-10 levels in the burn patients. However, our analysis of LPS stimulated whole blood from healthy controls provides limited support of the association between decreased levels of IL-10 and the IL-10 592A/−819T allele. The lack of association between IL-10 promoter genotype and risk for sepsis was unexpected, as sepsis was strongly linked with death (nonseptic patients experienced 9% mortality compared with 40% mortality among patients with sepsis). Furthermore, the majority of deaths were due to organ failure or septic shock. Presumably, the IL-10 promoter polymorphisms act to reduce mortality risk through a pathway that is at least partially independent of organ failure and septic shock. A second explanation is that the study lacked statistical power to resolve associations between IL-10 genotype and risk for complicated sepsis.
Although the finding of an allelic association alone is not expected to alter standard care, these findings are the first step toward resolving genetic predisposition for a complicated clinical course after traumatic injury. Ultimately, it is expected in the future that data of this type will allow clinicians to direct care toward the individual patient, rather than the disease state or injury.
ACKNOWLEDGMENTS
This study was supported by NIH grants 5P50 GM21681-42 and 5T32 GM008593-13.
SS SSupported by NIH/NIGMS 5P50 GM21681-43, 5T32 GM008593-13, 5K08GM071646-5.
REFERENCES
- 1.Miller SF, Bessey PQ, Schurr MJ, et al. National burn repository 2005: A 10-year review. J Burn Care Res. 2006;27:411. doi: 10.1097/01.BCR.0000226260.17523.22. [DOI] [PubMed] [Google Scholar]
- 2.Runyan CW, Casteel C, Perkis D, et al. Unintentional injuries in the home in the United States Part I: Mortality. Am J Prev Med. 2005;28:73. doi: 10.1016/j.amepre.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Merrell SW, Saffle JR, Larson CM, et al. The declining incidence of fatal sepsis following thermal injury. J Trauma. 1989;29:1362. doi: 10.1097/00005373-198910000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 5.McDaniel DO, Hamilton J, Brock M, et al. Molecular analysis of inflammatory markers in trauma patients at risk of post-injury complications. J Trauma. 2007;63:147. doi: 10.1097/TA.0b013e31806bf0ab. discussion 157. [DOI] [PubMed] [Google Scholar]
- 6.Arcaroli J, Fessler MB, Abraham E. Genetic polymorphisms and sepsis. Shock. 2005;24:300. doi: 10.1097/01.shk.0000180621.52058.e1. [DOI] [PubMed] [Google Scholar]
- 7.Barber RC, Aragaki CC, Rivera-Chavez FA, et al. TLR4 and TNF-alpha polymorphisms are associated with an increased risk for severe sepsis following burn injury. J Med Genet. 2004;41:808. doi: 10.1136/jmg.2004.021600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Keefe GE, Hybki DL, Munford RS. The G–>A Single Nucleotide Polymorphism at the −308 Position in the Tumor Necrosis Factor-alpha Promoter Increases the Risk for Severe Sepsis after Trauma. J Trauma. 2002;52:817. doi: 10.1097/00005373-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Mosser DM, Zhang X. Interleukin-10: New perspectives on an old cytokine. Immunol Rev. 2008;226:205. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amre DK, Mack DR, Morgan K, et al. Interleukin 10 (IL-10) gene variants and susceptibility for paediatric onset Crohn's disease. Aliment Pharmacol Ther. 2009;29:1025. doi: 10.1111/j.1365-2036.2009.03953.x. [DOI] [PubMed] [Google Scholar]
- 11.Hee CS, Gun SC, Naidu R, et al. Comparison of single nucleotide polymorphisms in the human interleukin-10 gene promoter between rheumatoid arthritis patients and normal subjects in Malaysia. Mod Rheumatol. 2007;17:429. doi: 10.1007/s10165-007-0612-9. [DOI] [PubMed] [Google Scholar]
- 12.Yao JG, Gao LB, Liu YG, et al. Genetic variation in interleukin-10 gene and risk of oral cancer. Clin Chim Acta. 2008;388:84. doi: 10.1016/j.cca.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Bagnoli S, Cellini E, Tedde A, et al. Association of IL10 promoter polymorphism in Italian Alzheimer's disease. Neurosci Lett. 2007;418:262. doi: 10.1016/j.neulet.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Lowe PR, Galley HF, Abdel-Fattah A, et al. Influence of interleukin-10 polymorphisms on interleukin-10 expression and survival in critically ill patients. Crit Care Med. 2003;31:34. doi: 10.1097/00003246-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Greenhalgh DG, Saffle JR, Holmes JH, et al. American Burn Association Consensus Conference to Define Sepsis and Infection in Burns. J Burn Care Res. 2007;28:776. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 16.Finnerty CC, Herndon DN, Przkora R, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 17.Csontos C, Foldi V, Palinkas L, et al. Time course of pro- and anti-inflammatory cytokine levels in patients with burns—prognostic value of interleukin-10. Burns. 2009;36:483. doi: 10.1016/j.burns.2009.10.009. [DOI] [PubMed] [Google Scholar]


