Abstract
Background & Aims
Isothiocyanates (ITCs) derived from cruciferous vegetables have been shown to be promising agents against cancer in human cell culture, animal models, and in epidemiological studies. Several epidemiological studies have demonstrated an inverse relationship between intake of dietary isothiocyanates and the risk of cancers, particularly lung, colon, and breast. More importantly, the protective effects of dietary ITCs appear to be influenced by glutathione S-transferase (GST) genotype; specifically, individuals with glutathione S-transferase theta 1 (GSTT1) and glutathione S-transferase Mu 1 (GSTM1) null are better protected than those with GSTT1 and M1 positive. Although majority of studies, especially those conducted in populations exposed to ITCs rich diet, demonstrated such effects there are a few studies showed opposite or no association. While evidence for the interactions of dietary ITCs with GST genes is relatively strong, the reasons for the differential effects remain unclear. In this study, we examined one possible mechanism whether subjects with the null genotypes excrete ITCs at a slower rate than those with the positive genotypes after drinking watercress juice, a rich source of ITCs.
Methods
A total of 48 subjects, 28 GSTT1 and M1 positive and 20 null genotypes were enrolled in the study. The rates of excretion were determined using five urine samples collected over a period of 24 hours after drinking watercress juice.
Results
No statistically significant differences in the rates of isothiocyanate excretion and the time of peak excretion were observed between the two tested groups with positive and null genotypes.
Conclusions
GSTT1 and M1 genotypes are not likely to be involved in the rate of excretion of ITCs in watercress. The demonstrated differences in protection among subjects with the two genotypes are not likely due to differences in overall ITC excretion rates, however, excretion rates of ITCs other than PEITC need to be investigated. Other yet to be identified mechanism(s) may underlie the diet and gene interactions between dietary ITCs and GST genotypes in human cancer prevention. Further research is needed to evaluate the protective mechanisms of isothiocyanates against cancer.
Keywords: isothiocyanates, watercress, PEITC, GST genotype, cancer prevention
Introduction
Isothiocyanates (ITCs) are a family of compounds found in cruciferous vegetables that have been shown to have promising chemopreventive activities (1). In animal and human cell culture studies, these compounds inhibit carcinogenesis in a number of tissues, including lung, breast, colon, and bladder epithelium (2–6). Epidemiological studies have also shown that intake of dietary ITCs are associated with reduced risks of certain human cancers (1, 7–15). In a cohort study in Shanghai, dietary ITC intakes were shown to be inversely associated with the risk of lung cancer (10). In that study, the intake of dietary ITCs was estimated by measuring the total ITC metabolites excreted in the urine as a validated biomarker (16, 17). More importantly, it showed that GSTM1 and T1 genotypes modulate the protective effects of dietary ITCs against lung cancer. The strongest protection was seen in individuals with GSTM1 and T1 null, whereas no protective effect was seen in GSTM1 and T1 positive individuals. Although the majority of studies support such observation, others found no differences in the protective effects of cruciferous vegetables between GSTM1/T1 positive and null individuals (14, 18). We address this disparity in greater details in the discussion.
Subsequent studies based on either urinary ITC levels or questionnaires not only confirmed the roles of GSTM1 and GSTT1 null genotypes in the effect of dietary ITCs against lung cancer (7, 15), but further showed that dietary ITC intake is associated with reduced risks of other cancers, such as breast and colon (8, 9, 11, 19). These studies demonstrated the importance of diet-gene interactions in protection against cancer. While the importance of GSTM1 and T1 null polymorphism in cancer prevention by dietary ITCs has been supported by a number of independent studies (9–11, 13–15, 18–25), the mechanisms for the differences related to GST null and positive genotypes are still not understood.
The metabolism of ITCs in humans has been investigated. A major route of metabolism is the conjugation of ITC with glutathione (GSH) followed by enzymatic degradation and N-acetylation via the mercapturic acid pathway (26). Because GSTs have been shown to mediate conjugation of GSH with ITC (12, 27), a plausible hypothesis for the GST gene-diet interaction is that individuals with the GSTT1 and M1 null genotypes have a slower overall rate of excretion of dietary ITCs and, consequently, more sustained bioavailability as compared with those who are GSTT1 and M1 positive genotypes (28).
To test this hypothesis and further define the role of dietary ITCs in cancer prevention, we conducted a trial to determine whether individuals with both GSTT1 and GSTM1 null genotypes have lower rates of ITC excretion than those with positive genotypes by quantifying total ITC metabolites in the urine over a period of 24 h after drinking watercress juice which is rich in phenethyl isothiocyanate (PEITC).
Materials and Methods
Chemicals
1,2-Benzenedithiol was purchased from Fluka (Sigma-Aldrich Co., St. Louis, MO). L-Ascorbic acid was purchased from Aldrich (Sigma-Aldrich Co., St. Louis, MO). The N-acetylcysteine conjugate of phenethyl isothiocyanate (PEITC-NAC) used was synthesized in our laboratory (29). All other chemicals used were HPLC or ACS reagent grade.
Watercress juice
Watercress juice was prepared by blending watercress from a local grocery store and tap water in proportion of 1:4 (w/w). After blending in a mechanical blender for 1 min. at high speed, the resulting suspension was filtered through two layers of cheesecloth. The filtered juice was kept at 4° C until use. Fresh juice was prepared for each treatment and 18 separated preparations were made during the study period. The juice preparations for most of the treatments were stored for approximately 2 h before treatment. However, for reasons of convenience, preparations were made late Fridays or during weekends for those treatments that occurred early morning of the following Monday.
Study subjects
Recruitment and screening criteria
All subjects participating in this study gave informed consent before treatment. The study protocol was approved by MedStar Research Institute-Georgetown University Oncology Institutional Review Board. Subjects who responded to the on-campus flyers were contacted for initial questionnaire-based screening for eligibility. The criteria of eligibility were as follows: age between 18 and 60 years old, HIV negative, no active prescription medications, non-tobacco users, non-diabetic, not currently pregnant or breast feeding, and having GSTM1 and GSTT1 positive or GSTM1 and GSTT1 null genotypes. In the first phase of the study, genotyping of GSTM1 and GSTT1 was done on eligible subjects. Those who had both GSTM1 and GSTT1 positive or GSTM1 and GSTT1 null genotypes were contacted. The subjects who agreed to participate in the second phase of the study were scheduled to come to the General Clinical Research Center at Georgetown University Hospital to be treated with watercress juice and donate urine samples for the next 24 hours.
Treatment protocol
Watercress juice drinking
The majority of urinary ITCs are excreted within the first 16 h after ingestion and the excretion is nearly complete 24 h after, although the total ITC excretion can last as long as 72 h (12, 30, 31). Subjects were instructed to avoid any food containing ITCs for 72 h before treatment. A detailed list of foods containing ITCs was provided to each subject (please see supplementary materials section). The night before treatment the subjects were asked to avoid any food and drink after midnight. In the morning of treatment day, subjects drank 200 ml of watercress juice. Immediately after drinking the juice, a breakfast consisting of 8 ounces of orange juice, one bagel with a pat of butter, a bowl of cornflakes with skim milk, and coffee, if desired, was served. During the remaining 24 h of urine collection period, subjects were free to eat whatever they chose as long as they avoided the ITC-containing foods on the list.
Blood and Urine collections
Blood samples were drawn at Georgetown University General Clinical Research Center by trained nurses. After processing and separation of blood components, buffy coat samples were stored at −80° C for further assays. Blood was drawn once after the initial screening phase purely for genotyping purposes.
A morning urine sample was collected prior to treatment (baseline). After drinking watercress juice, five samples of total urine were collected from 0–2, 2–4, 4–8, 8–12 and 12–24 hours. Each sample was collected in separate jars containing 0.5 g of ascorbic acid as a preservative as previously described (9). The total volume of each collection was recorded and small aliquots (3 × 1.8 ml) of urine were taken and kept at −80° C until analysis.
Genotyping
GSTM1 and GSTT1 genotypes were determined by a multiplex polymerase chain reaction (PCR) using genomic DNA as previously described (32). Genotyping was performed by the 5’ nuclease assay (TaqMan) using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with the constitutively present gene BRCA1 as an internal positive control. Genotyping was performed by laboratory personnel blinded to the treatment arm, and a random 10% of the samples were repeated for validation. Two lab personnel independently reviewed all genotyping results, and samples with unclear results were rerun until 100% concordance was achieved.
HPLC analysis of total ITC metabolites in urine and ITCs content in watercress juice
Urine analysis
The urine samples were analyzed by HPLC after reacting with 1,2-benzenedithiol (BDT) as previously described (8) with minor revisions: an ITC-free urine sample was used for PEITC-NAC standards (0, 0.5, 1, 2, 5, 10, 15, 20, 25 and 50 mM) instead of 20 mM phosphate buffer (pH 5.0); the 1 mM BDT in 2-propanol was used instead of 10 mM for the cyclocondensation reaction to minimize byproducts formation; in the HPLC assay the mobile phase consisted of a mixture of methanol and water 5:2 (v/v) rather than the previously reported ratio of 7:3 (v/v) due to the technical design of the gradient controller. In every assay samples containing mobile phase and standard in triplicates were interspersed at every nine samples throughout the batch for quality control of the assay.
For each group of samples used for quality control, a different concentration of standard was used (5, 10 and 15 µM). For each set of samples, average ITC concentration, standard deviation and coefficient of variation (CV) were calculated. The triplicate values were then checked for consistency. The criteria used were: 25% or less CV for ITC values between 0.5 to 2 µM and 15% CV for values above 2 µM. Samples that did not fit these criteria were re-analyzed. To ensure assay reproducibility 3% of randomly selected samples were re-analyzed. The average intra- and inter-batch CV for triplicates was 4% and 5%, respectively.
Analysis of watercress juice
Watercress juice was centrifuged for 15 min. (× 4,500 rpm) on a desktop Eppendorf 5804 centrifuge (Eppendorf, Madison, WI) and the supernatant was diluted 1:2 with water, then processed and analyzed in the same manner as urine samples. Because watercress juice was prepared with water, all standard solutions used in the assay were prepared using water instead of ITC-free urine.
Statistical methods
Demographic characteristics, specifically gender and race, were compared between the GST positive and null groups using Fisher’s exact test. In addition, t-tests were used to compare age at juice consumption, urinary baseline ITC level, and ITC content in juice between the groups. Normality assumptions were checked using graphical techniques on the residuals. Several approaches were used to examine differences in magnitude of ITC excretion between GST positive and null groups.
The primary outcome was the cumulative amount of ITC excreted over time, which was calculated by adding the total amount of ITC excreted by the end of each interval to the amount excreted in all previous intervals. This resulted in five measurements per subject over the collection period.
Secondary outcomes included the total amount of ITC excreted per two hours and the time at which the peak excretion occurred. Since the time intervals between collections were not equal and we expected more ITC excretion over longer time periods, the total amount of ITC excreted was standardized to a two hour interval. This assumes that ITC was excreted at a uniform rate over the entire interval, but allows the measurements from different collections to be comparable. The ITC content in juice, urine baseline ITC (amount of ITC metabolites in urine collected before the treatment), and demographics were considered as covariates.
Since ITC excretion was measured repeatedly on the same subject over time, we expected that the measurements from the same subject were correlated. In order to account for the dependence, a mixed effects model was used to determine if there was a difference in cumulative ITC excretion between the GST positive and null groups over time. The model building began with time, GST status, and their interaction in the model. Since cumulative ITC excretion follows a nonlinear trajectory, higher order terms with time and their interactions with GST status, urine baseline ITC, and ITC content in juice were introduced to the model. The overall rate of cumulative ITC excretion between GST groups was compared using a likelihood ratio test in which all terms involving the interaction between GST status and time were tested jointly. A similar modeling approach was used for the total amount of ITC excreted per two hours after consumption. The total amount was transformed using the natural logarithm in order to meet normality assumptions. The results were back-transformed and are presented on the original scale. In addition, Fisher’s exact test was used to compare the time interval at which the peak ITC excretion occurred between the GST groups. Finally, a sensitivity analysis was performed by excluding the ITC measurements after an unusual spike in total ITC excreted. The same analysis was repeated and compared to determine the impact that these outliers may have had on the results.
The study planned to enroll 25 subjects in each of the two groups in order to have an 80% chance of detecting as significant (at the two-sided 5% level) a 12 unit difference in mean ITC excretion in any time interval, with an assumed standard deviation of 15. Statistical analyses were performed using SAS software, version 9.1 (SAS Institute Inc., Cary, NC).
Results
Out of 417 subjects who responded to the posted flyers, 174 were eligible for the study. After genotyping, 52 were found to have both GSTM1 and GSTT1 positive and 26 were negative for both. Of the 78 subjects with the desired genotypes, 48 subjects participated in the trial, 28 GST1 and M1 positive and 20 GST1 and M1 null. Of the GST positive subjects 39% were males compared to 50% in the GST null group (Table 1). Among those reporting race (63%), 70% were white, 20% Asian, 3% black and 7% defined themselves as “other”. There was no statistically significant difference between the two groups on gender or race (p=0.56 and 0.99, respectively). The average age was 26 years and there were no significant differences between GST positive and null subjects (p=0.07) in age. The concentration of ITC in the juice had an overall average of 72 µM and ranged between 15 and 206 µM. This wide range of ITC content in the juice was caused by a small number of subjects consuming juices containing relatively high levels of ITCs (91 – 206 µM) possibly due to batch differences in watercress purchased from the store. In addition, the significant differences of ITC content in the juice ingested by the subjects may also be caused by the unexpected, rapid decomposition of ITCs at 4°C during the first few hours after preparation (data not shown). Subsequently, to minimize the variability, we collected and immediately froze the juice samples used at the time of sampling. Both factors may contribute to the statistically significant difference in the ITC content between GST positive and GST null subjects (p=0.02) (Table 1). There was no statistically significant difference in baseline ITC in the urine between the two groups (p=0.22).
Table 1.
Characteristics of subjects by GSTT1 and M1 status (N=48), gender, race, age at the time of study, and juice concentration used.
| GSTT1 and M1 | |||
|---|---|---|---|
| Null (N=20) a |
Positive (N=28) |
p-value | |
| N (%) | N (%) | ||
| Gender | 0.56 | ||
| Male | 10 (50%) | 11 (39%) | |
| Female | 10 (50%) | 17 (61%) | |
| Race b | 0.99 | ||
| White | 6 (67%) | 15 (71%) | |
| Non-White | 3 (33%) | 6 (29%) | |
| Mean ± SD | Mean ± SD | ||
| Age, years | 29 ± 11 | 25 ± 5.8 | 0.07 |
| ITC concentration in juice [µM] c | 46 ± 45 | 88 ± 60 | 0.02 |
| Baseline ITC [µmol] d | 0.83 ± 1.64 | 0.42 ± 0.61 | 0.22 |
Total number of subjects.
Unknown: 11 GST null, 7 GST positive.
Unknown: 3 GST null.
Amount of ITC in the urine sample collected over 24 h before watercress juice treatment.
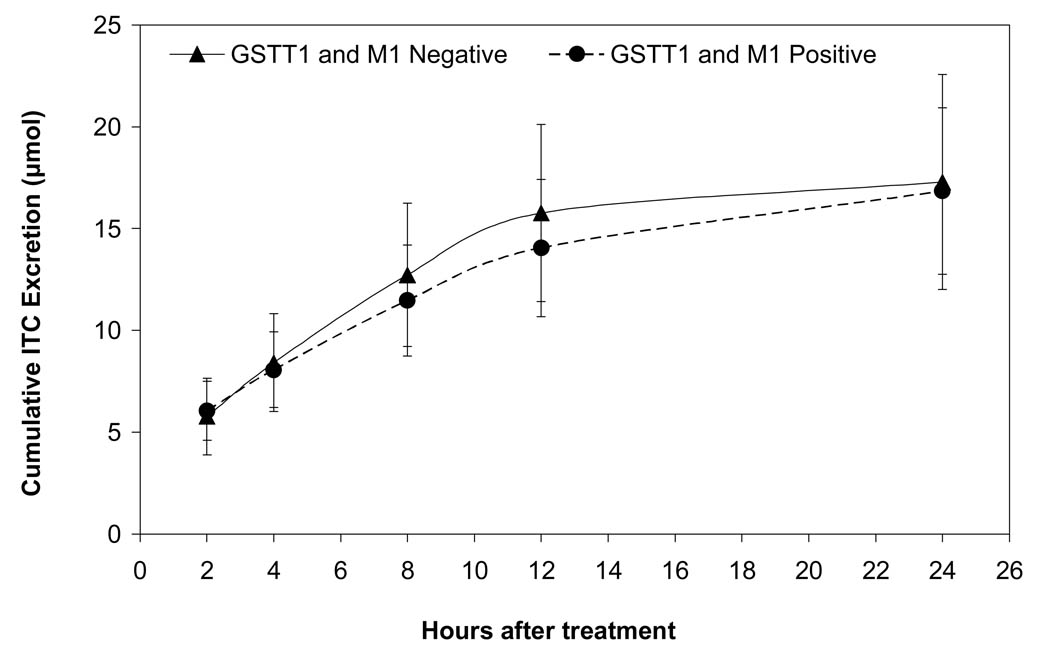
The final model to estimate the trend of cumulative ITC excretion over the 24 h follow-up period included fixed effects for time, GST status, a quadratic time term to model the curvature in the cumulative ITC trajectory, and the interactions between GST status and time and ITC content in juice. The GST positive group consumed juice with a higher concentration; juice concentration remained in the final model to adjust for this difference. Results did not change when other functional forms for juice concentration were considered such as log-transformed concentration or adding a quadratic term into the model. In addition, age was removed from the final model since it was not a significant predictor (p=0.21). Also, random effects for intercept, time and quadratic time were included in order to account for the correlation between measurements of ITC excretion on the same subject. The likelihood ratio test for the overall difference in rate of the excretion between the groups was not statistically significant after controlling for ITC content in juice (p=0.43). The estimated mean cumulative ITC excretion from the model among the GST null group was higher at the end of each collection interval except the first, after controlling for ITC content in juice. However, these differences were not statistically significant (Table 2). Figure 1 shows cumulative ITC excretion over time for subjects after consuming watercress juice.
Table 2.
Fitted mean cumulative ITC (µmol) excreted at each time point based on GSTT1 and M1 genotypes from subjects after consuming watercress juice with an ITC concentration of 72 µM from the linear mixed model (N=45).
| GSTT1 and M1 | |||
|---|---|---|---|
| Time (h) | Null | Positive | p-value of difference |
| 2 | 5.8 (3.8, 7.7) | 6.0 (4.6, 7.5) | 0.83 |
| 4 | 8.4 (5.9, 11) | 8.1 (6.2, 10) | 0.83 |
| 8 | 13 (9.1, 16) | 12 (8.7, 14) | 0.59 |
| 12 | 16 (11, 20) | 14 (11, 17) | 0.55 |
| 24 | 17 (12, 23) | 17 (13, 21) | 0.90 |
Figure 1.
Fitted mean cumulative ITC (µmol) (and 95% CI) excreted in the urine over 24 hours after drinking watercress juice for GSTT1 and M1 positive and null subjects with an ITC concentration of 72 µM estimated with a linear mixed model. The final model included fixed effects for time, GST status, a quadratic time term to model the curvature in the cumulative ITC trajectory, and the interactions between GST status and time and ITC content in juice and random effects for intercept, time, and quadratic time.
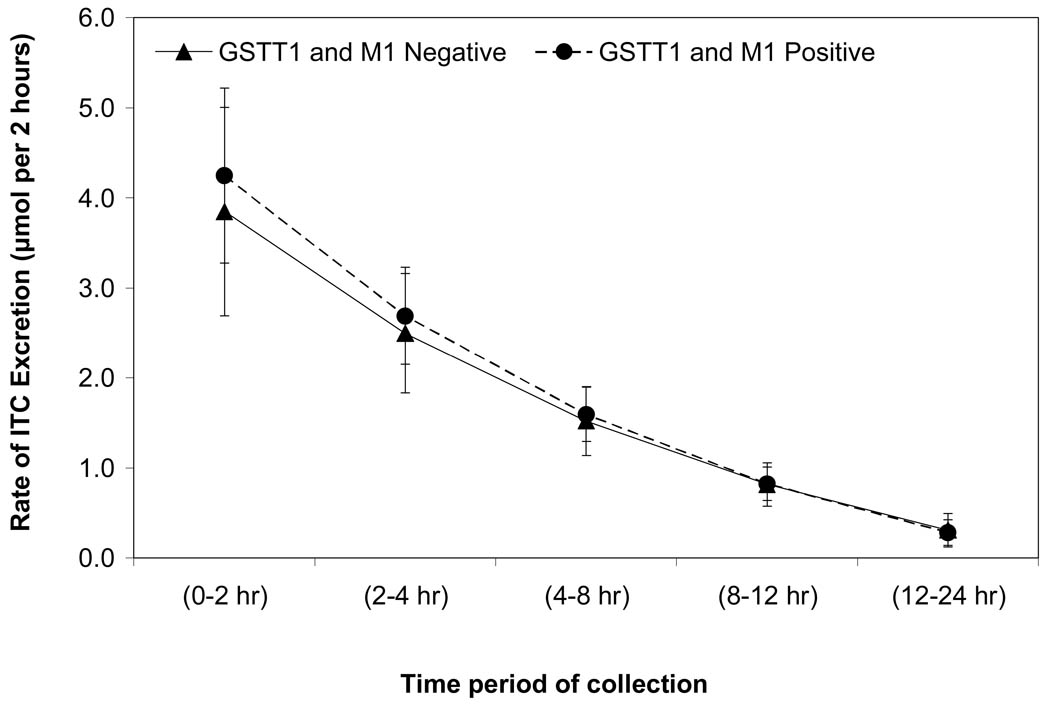
The mixed model for log-transformed total amount of ITC excretion included fixed effects for GST status, time, their interaction, juice content, and random effects for the intercept and slope. There was no statistically significant difference in the rate of excretion (slope) between the GST null and positive groups after controlling for ITC content in juice (p=0.53, Figure 2). Finally, there was no statistically significant difference in the time of peak excretion between the two groups (p=0.17).
Figure 2.
Fitted mean total ITC (and 95% CI) excreted in the urine for GSTT1 and M1 positive and null subjects with an ITC concentration of 72 µM estimated with a linear mixed model. The model was fit to the log-transformed total ITC values to meet modeling assumptions, however, the fitted means presented here have been back-transformed and are on the original scale. The final model included fixed effects for GST status, time, their interaction, juice content, and random effects for the intercept and slope.
Twenty five subjects were suspected of having consumed ITC-containing foods based on a spike in the total amount of ITC excreted during the follow-up period and had at least some outlying data removed for the sensitivity analysis. However, the results were robust to the apparent protocol violations. Since the conclusion did not change after these exclusions, the results presented here included ITC measurements for all the urine samples collected. The difference in cumulative ITC, the rate of ITC excretion, and the time to peak excretion between the groups remained statistically insignificant (p=0.58, 0.93, and 0.57, respectively).
Discussion
This study was designed to examine whether metabolic rates of dietary ITCs may explain the observations that GST1 and M1 null genotypes are better protected by cruciferous vegetables. The study showed that there are no significant differences in overall rate, total amount of excretion, and the time of peak excretion of ITCs after drinking watercress juice between the null and positive GSTT1 and M1 genotypes and the results point to possibilities that mechanisms other than PEITC metabolism may be involved.
The major route of metabolism of ITCs in humans involves the initial conjugation with GSH followed by enzymatic degradation via the mercapturic acid pathway (12, 27, 30). In human urine, the N-acetylcysteine conjugates of ITCs have been found to be the predominant metabolite. Excretion peaks between 1–2 h after ingesting broccoli, watercress or its juice and is intake-dependent (12, 16, 31). An HPLC-based assay has been used to quantify total urinary ITCs, including the N- acetylcysteine conjugates, as a marker of dietary ITC intake by converting to a cyclocondensation product with 1,2-benzenedithiol (16). Approximately, a total of 75% of all ITC ingested is excreted within 8 h. The urinary marker of dietary ITCs has been validated against questionnaire responses in a Singapore cohort study and then applied to numerous population studies to investigate the role of dietary ITCs in human cancers (8–10, 15, 17, 20).
Several studies have shown a positive association between GSTM1 and GSTT1 null genotypes and cancer prevention by dietary ITCs (8–11, 15, 19). The Shanghai cohort study was the first to link dietary ITC intake to a decreased risk of lung cancer and showed that the protective effect of ITCs was seen only among individuals with homozygous deletion of either GSTM1 or GSTT1, and that it was particularly strong in individuals with deletion of both GSTM1 and GSTT1 (10). This conclusion is supported by a large case-control study of patients with lung cancer conducted among mostly non-smoking Chinese women in Singapore (15). A large prospective study from central and eastern Europe further lends support by confirming a protective effect of cruciferous vegetables against lung cancer, and only individuals with GSTM1 null or both GSTM1/T1 null were protected (7). Recently, taking into account results from 6 cohort and 12 case-control studies, a review concluded that, independent of cigarette smoking, there is a modest inverse association between lung cancer risk and high intake of cruciferous vegetables and that the effect is stronger among subjects with null genotype for GSTM1 and GSTT1 (33).
Similar conclusions were made in a case-control study of breast cancer conducted in Shanghai and a colon cancer study in Los Angeles (8, 9, 18, 23, 24). In another report, low ITC intake and GSTM1 and GSTT1 null genotypes were shown to be associated with increased lung cancer risk, whereas subjects with deficient genotypes and high ITC intake did not have increased lung cancer risk (13).
Contrary to those findings, one study conducted in the US showed a protective effect of cruciferous vegetable against lung cancer only in individuals with GSTM1 positive genotype (14). In a Dutch case-control colorectal adenoma study, no differences were found in the protective effect of cruciferous vegetables between GSTM1/T1 positive and null individuals; however, less active GSTP1 and GSTA1 genotypes seemed associated with the protective effect of ITC intake (18).
The divergent results may be related to substantial differences in dietary habits between populations. Possible explanations in the discrepancy are frequency of consumption, types of cruciferous vegetable consumed, and possibly differences in GST specificities towards various ITCs consumed by each populations (22, 27). For example, the daily estimated consumption of cruciferous vegetables in North America was reported to be 25–30 g, with a range of 16–40 g, significantly lower compared to those studies conducted in Shanghai, China (more than 100 g per day) and the general Asian region (40–80 g per day) (1). Furthermore, the food questionnaire used in most of those studies is not as reliable as urinary ITCs metabolites, especially when the consumption is relatively low.
Because GSTs can mediate the conjugation of ITC with GSH, a plausible explanation for the protective effect in GSTM1 and GSTT1 null genotypes is that they have more sustained bioavailability of ITCs due to slower excretion (8, 10, 15, 17). An earlier study showed that, while GSTM1 null and positive individuals have no differences in urinary ITC levels, GSTT1 positive is significantly associated with increased total urinary ITC excretion, especially in subjects having high consumption (17). However, Gasper et al. reported that GSTM1 null subjects excreted sulforaphane (SFN) metabolites at a faster rate during the first 6 h and to a greater extent during 24 h than GSTM1 positive subjects after consumption of broccoli (22).
Contrary to that report, Steck et al. showed no differences in the average 24 h urinary excretion of ITC between groups with the different GST genotypes, except that a greater proportion of individuals with GSTM1 null genotype had a higher than median urinary ITC level when compared to those with GSTM1 positive (20). However, as only a 24 h urine sample was collected in that study, it may have failed to capture any possible differences in the rate of excretion between the positive and null GST genotypes due to the relatively fast ITC metabolism. Furthermore, the number of subjects with GSTM1/T1 null vs. positive genotypes seems quite low. It should also be noted that there are significant differences in GST activity toward SFN vs. PEITC. SFN has been shown to be a poor substrate for all GST enzymes with significantly slower GSH conjugation by GSTM1 than other ITCs (22, 27). While these findings are plausible explanations for the discrepancies from the previous studies, the inconsistent results prompted us to conduct a more detailed study on kinetics of excretion after drinking watercress juice containing known amounts of ITCs. In our study, we determined the overall rates of ITC excretion by collecting multiple urine samples over a 24 h period after consumption. Considering the rapid ITC metabolism (12, 31, 34), it is possible that the earlier time points may reflect more accurately the relationship between GST genotype and the ITC metabolic rate.
Our results showed that there are no significant differences in overall rate, total amount of excretion, and the time of peak excretion of ITCs after drinking watercress juice between the null and positive GSTT1 and M1 genotypes, however, this study has some limitations. Even though we used the same quantity of fresh watercress for juice preparation for each subject we did observe significant variations in the amount of ITC in the watercress juice consumed due to unexpected rapid decomposition under the storage conditions, and possibly cultivar differences of watercress batches from store. Also, the watercress juice administrated was not corrected by BMI or other related factors. Although the excretion was based on the amount of ITC measured in the ingested juice, it may still be argued, with these limitations, that a larger sample size may be needed to detect any differences in the ITC metabolism by these two genotype groups. Nevertheless, we believe the differences, if they exist, may be small.
Several possible explanations may be suggested for the lack of difference in these two genotypes. First, GSTT1 and M1 do not play a significant role as a rate determinant step in ITC conjugation; second, PEITC, a major component in watercress, is not a good substrate for GSTT1 and M1; and third, genes other than GST in the mercapturic acid pathway may be more important in metabolizing ITCs (35, 36). The question is why individuals with GSTT1 and M1 null genotypes are better protected.
The results of the present study suggest that mechanism(s) other than ITC metabolism may exist for the observed protection in GSTT1 and M1 null genotypes. For example, ITC metabolism is differentially regulated in target tissues such as lung, breast and colon. The lack of GSTT1 and M1 genes may influence the expression of other genes that are associated with reduced risk of cancer. Earlier studies have shown that GSTM1 genotype status may influence other biotransformation enzymes including GSTA, GSTM and cytochrome P450 1A2 (28, 37). A recent study showed that GSTT1 activities can be up-regulated by GSTM1 null genotypes upon exposure to pesticides (38). It is plausible that genes other than GST family members or cytochrome P450s may be modulated by dietary ITCs in the absence of GSTM1 and T1 genes. More studies are needed to examine these possibilities in order to fully understand the proficient protection toward GSTT1 and M1 null genotypes by dietary ITCs.
Supplementary Material
Acknowledgments
This work was supported by NIH / National Cancer Institute R01 CA 100853-05 grant. We thank the staff of the Georgetown University Medical Center General Clinical Research Center for their substantial help in conducting the clinical work. We also thank Judy Chung and Karen Howenstein for their efforts in recruitment and preparing watercress juice. The specific contribution of each author to the work: MD carried out the studies, samples, data analyses and drafted the manuscript. AW and AMN performed statistical analysis and helped to draft the manuscript. DG and PS performed genotyping and helped to draft the manuscript. YLZ participated in data analysis and helped draft manuscript. RR participated in study design and manuscript preparation. FLC conceived of the study, and participated in its design and coordination and drafted the manuscript. All authors read and approved the final manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
There are no conflicts of interest to disclose.
Reference List
- 1.WHO. Lyon (France): IARC; IARC Handbook on Cancer Prevention Vol. 9: Cruciferous vegetables, isothiocyanates and indoles. 2004
- 2.Chiao JW, Chung FL, Kancherla R, Ahmed T, Mittelman A, Conaway CC. Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int J Oncol. 2002;20(3):631–636. doi: 10.3892/ijo.20.3.631. [DOI] [PubMed] [Google Scholar]
- 3.Yang YM, Conaway CC, Chiao JW, Wang CX, Amin S, Whysner J, Dai W, Reinhardt J, Chung FL. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002;62(1):2–7. [PubMed] [Google Scholar]
- 4.Conaway CC, Wang CX, Pittman B, Yang YM, Schwartz JE, Tian D, McIntee EJ, Hecht SS, Chung FL. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65(18):8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 5.Pappa G, Lichtenberg M, Iori R, Barillari J, Bartsch H, Gerhauser C. Comparison of growth inhibition profiles and mechanisms of apoptosis induction in human colon cancer cell lines by isothiocyanates and indoles from Brassicaceae. Mutat Res. 2006;599(1–2):76–87. doi: 10.1016/j.mrfmmm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Munday R, Mhawech-Fauceglia P, Munday CM, Paonessa JD, Tang L, Munday JS, Lister C, Wilson P, Fahey JW, Davis W, Zhang Y. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008;68(5):1593–1600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 7.Brennan P, Hsu CC, Moullan N, Szeszenia-Dabrowska N, Lissowska J, Zaridze D, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Gemignani F, Chabrier A, Hall J, Hung RJ, Boffetta P, Canzian F. Effect of cruciferous vegetables on lung cancer in patients stratified by genetic status: a mendelian randomisation approach. Lancet. 2005;366(9496):1558–1560. doi: 10.1016/S0140-6736(05)67628-3. [DOI] [PubMed] [Google Scholar]
- 8.Fowke JH, Chung FL, Jin F, Qi D, Cai Q, Conaway C, Cheng JR, Shu XO, Gao YT, Zheng W. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003;63(14):3980–3986. [PubMed] [Google Scholar]
- 9.Fowke JH, Shu XO, Dai Q, Shintani A, Conaway CC, Chung FL, Cai Q, Gao YT, Zheng W. Urinary isothiocyanate excretion, brassica consumption, and gene polymorphisms among women living in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2003;12(12):1536–1539. [PubMed] [Google Scholar]
- 10.London SJ, Yuan JM, Chung FL, Gao YT, Coetzee GA, Ross RK, Yu MC. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: a prospective study of men in Shanghai, China. Lancet. 2000;356(9231):724–729. doi: 10.1016/S0140-6736(00)02631-3. [DOI] [PubMed] [Google Scholar]
- 11.Seow A, Vainio H, Yu MC. Effect of glutathione-S-transferase polymorphisms on the cancer preventive potential of isothiocyanates: an epidemiological perspective. Mutat Res. 2005;592(1–2):58–67. doi: 10.1016/j.mrfmmm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev. 2001;10(5):501–508. [PubMed] [Google Scholar]
- 13.Spitz MR, Duphorne CM, Detry MA, Pillow PC, Amos CI, Lei L, de Andrade M, Gu X, Hong WK, Wu X. Dietary intake of isothiocyanates: evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9(10):1017–1020. [PubMed] [Google Scholar]
- 14.Wang LI, Giovannucci EL, Hunter D, Neuberg D, Su L, Christiani DC. Dietary intake of Cruciferous vegetables, Glutathione S-transferase (GST) polymorphisms and lung cancer risk in a Caucasian population. Cancer Causes Control. 2004;15(10):977–985. doi: 10.1007/s10552-004-1093-1. [DOI] [PubMed] [Google Scholar]
- 15.Zhao B, Seow A, Lee EJ, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Yu MC, Lee HP. Dietary isothiocyanates, glutathione S-transferase -M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev. 2001;10(10):1063–1067. [PubMed] [Google Scholar]
- 16.Chung FL, Jiao D, Getahun SM, Yu MC. A urinary biomarker for uptake of dietary isothiocyanates in humans. Cancer Epidemiol Biomarkers Prev. 1998;7(2):103–108. [PubMed] [Google Scholar]
- 17.Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, Coetzee GA, Yu MC. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev. 1998;7(9):775–781. [PubMed] [Google Scholar]
- 18.Tijhuis MJ, Wark PA, Aarts JM, Visker MH, Nagengast FM, Kok FJ, Kampman E. GSTP1 and GSTA1 polymorphisms interact with cruciferous vegetable intake in colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2943–2951. doi: 10.1158/1055-9965.EPI-05-0591. [DOI] [PubMed] [Google Scholar]
- 19.Seow A, Yuan JM, Sun CL, Van Den BD, Lee HP, Yu MC. Dietary isothiocyanates, glutathione S-transferase polymorphisms and colorectal cancer risk in the Singapore Chinese Health Study. Carcinogenesis. 2002;23(12):2055–2061. doi: 10.1093/carcin/23.12.2055. [DOI] [PubMed] [Google Scholar]
- 20.Steck SE, Gammon MD, Hebert JR, Wall DE, Zeisel SH. GSTM1, GSTT1, GSTP1, and GSTA1 polymorphisms and urinary isothiocyanate metabolites following broccoli consumption in humans. J Nutr. 2007;137(4):904–909. doi: 10.1093/jn/137.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Kolm RH, Mannervik B, Talalay P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem Biophys Res Commun. 1995;206(2):748–755. doi: 10.1006/bbrc.1995.1106. [DOI] [PubMed] [Google Scholar]
- 22.Gasper AV, Al Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82(6):1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 23.Lin HJ, Probst-Hensch NM, Louie AD, Kau IH, Witte JS, Ingles SA, Frankl HD, Lee ER, Haile RW. Glutathione transferase null genotype, broccoli, and lower prevalence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 1998;7(8):647–652. [PubMed] [Google Scholar]
- 24.Slattery ML, Kampman E, Samowitz W, Caan BJ, Potter JD. Interplay between dietary inducers of GST and the GSTM-1 genotype in colon cancer. Int J Cancer. 2000;87(5):728–733. [PubMed] [Google Scholar]
- 25.Joseph MA, Moysich KB, Freudenheim JL, Shields PG, Bowman ED, Zhang Y, Marshall JR, Ambrosone CB. Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr Cancer. 2004;50(2):206–213. doi: 10.1207/s15327914nc5002_11. [DOI] [PubMed] [Google Scholar]
- 26.Brusewitz G, Cameron BD, Chasseaud LF, Gorler K, Hawkins DR, Koch H, Mennicke WH. The metabolism of benzyl isothiocyanate and its cysteine conjugate. Biochem J. 1977;162(1):99–107. doi: 10.1042/bj1620099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolm RH, Danielson UH, Zhang Y, Talalay P, Mannervik B. Isothiocyanates as substrates for human glutathione transferases: structure-activity studies. Biochem J. 1995;311(Pt 2):453–459. doi: 10.1042/bj3110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lampe JW, Chen C, Li S, Prunty J, Grate MT, Meehan DE, Barale KV, Dightman DA, Feng Z, Potter JD. Modulation of human glutathione S-transferases by botanically defined vegetable diets. Cancer Epidemiol Biomarkers Prev. 2000;9(8):787–793. [PubMed] [Google Scholar]
- 29.Jiao D, Conaway CC, Wang MH, Yang CS, Koehl W, Chung FL. Inhibition of N-nitrosodimethylamine demethylase in rat and human liver microsomes by isothiocyanates and their glutathione, L-cysteine, and N-acetyl-L-cysteine conjugates. Chem Res Toxicol. 1996;9(6):932–938. doi: 10.1021/tx9502094. [DOI] [PubMed] [Google Scholar]
- 30.Getahun SM, Chung FL. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol Biomarkers Prev. 1999;8(5):447–451. [PubMed] [Google Scholar]
- 31.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316(1–2):43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 32.Covault J, Abreu C, Kranzler H, Oncken C. Quantitative real-time PCR for gene dosage determinations in microdeletion genotypes. Biotechniques. 2003;35(3):594–596. 598. doi: 10.2144/03353dd02. [DOI] [PubMed] [Google Scholar]
- 33.Lam TK, Gallicchio L, Lindsley K, Shiels M, Hammond E, Tao XG, Chen L, Robinson KA, Caulfield LE, Herman JG, Guallar E, Alberg AJ. Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev. 2009;18(1):184–195. doi: 10.1158/1055-9965.EPI-08-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji Y, Morris ME. Determination of phenethyl isothiocyanate in human plasma and urine by ammonia derivatization and liquid chromatography-tandem mass spectrometry. Anal Biochem. 2003;323(1):39–47. doi: 10.1016/j.ab.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Shokeer A, Mannervik B. Minor modifications of the C-terminal helix reschedule the favored chemical reactions catalyzed by theta class glutathione transferase T1-1. J Biol Chem. 2010;285(8):5639–5645. doi: 10.1074/jbc.M109.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y. Molecular mechanism of rapid cellular accumulation of anticarcinogenic isothiocyanates. Carcinogenesis. 2001;22(3):425–431. doi: 10.1093/carcin/22.3.425. [DOI] [PubMed] [Google Scholar]
- 37.Lampe JW, King IB, Li S, Grate MT, Barale KV, Chen C, Feng Z, Potter JD. Brassica vegetables increase and apiaceous vegetables decrease cytochrome P450 1A2 activity in humans: changes in caffeine metabolite ratios in response to controlled vegetable diets. Carcinogenesis. 2000;21(6):1157–1162. [PubMed] [Google Scholar]
- 38.Fuciarelli M, Caccuri A, De Francesca M, Ferazzoli F, Piacentini S, Porreca F. Modulation of the GSTT1 activity by the GSTM1 phenotype in a sample of Italian farm-workers. Arch Toxicol. 2008 doi: 10.1007/s00204-008-0334-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.