Abstract
The evolution and persistence of cooperative social units depends on the ability to distinguish group members from nonmembers. The precision of discrimination, in turn, relies on variation in the labels that individuals use to recognize group members. However, this same variation can be selected against if individuals that are rejected as nonmembers incur a high cost. Here we provide evidence that selection against individuals from genetically diverse groups has contributed to the formation of the unicolonial colony structure that characterizes introduced populations of the invasive Argentine ant (Linepithema humile). Studies in both the laboratory and the field showed that individuals from less genetically diverse colonies attack individuals from more diverse colonies and that attackers survived agonistic encounters more than six times as often as recipients of aggression. This selection, in concert with reductions in genetic diversity after a founder event, likely creates a barrier to the establishment of new, genetically diverse introductions from the native range and may reduce genetic diversity within established populations in the introduced range.
The organization of individuals into cooperative social groups is one of the major transitions in the history of life (1). Sociality allows group members to increase their direct fitness through behaviors such as the division of labor, coordinated foraging, or cooperative breeding (2). Additionally, when social groups consist of relatives, individuals that direct altruistic behavior toward group members can also increase their indirect fitness (3–5). To realize these benefits of sociality, however, individuals must be able to distinguish precisely between group members and nonmembers. Consequently, natural selection has favored the evolution of a wide variety of recognition systems that regulate the expression of behaviors ranging from intense aggression to lifelong pair bonds, are built on different sensory modalities, and use a diverse array of signals and decision rules (6, 7).
Many social insects use odor cues (labels) to distinguish colony members from nonmembers (refs. 7–13; see below). These labels identify each individual's colony of origin and are used to form a recognition template against which it compares labels of conspecifics (7–13). A mismatch between an individual's template and the label of another individual generally triggers rejection (7–13). When odor cues are genetically based, the precision of recognition fundamentally depends on the levels of polymorphism and allelic frequencies at loci conferring these labels. For example, when two individuals share a label that is common in a population, there is a high probability that the allele is shared by chance, rather than by descent. However, as the number and rarity of alleles increases, so, too, will the likelihood that individuals sharing an allele will be closely related (or members of the same colony) (14). Consequently, negative (or inverse) frequency-dependent selection will favor the proliferation of rare alleles and the maintenance of a balanced polymorphism at recognition loci (9, 14, 15).
As first suggested by Crozier (16–19), however, a paradox can arise when rejection involves aggressive, physically damaging attacks on the rejected individuals. Because individuals with rare labels should be rejected more often than individuals with common labels, they will also incur the costs associated with rejection (e.g., energetic costs, injury, or death) more often (16–19). Moreover, when colony members share labels, individuals from high-diversity colonies should be rejected more frequently than individuals from low-diversity colonies. Similarly, when colony members share a template, individuals from high-diversity colonies should express rejection behavior less frequently than individuals from low-diversity colonies. When summed across many individuals within a colony, the costs of being aggressively rejected may affect colony-level attributes, such as foraging, recruitment, or colony growth. This asymmetric aggression can produce runaway positive frequency-dependent selection that reduces the very same polymorphism required for precise recognition.
Recent studies have speculated that this type of selection may operate in colonies of invasive ants (20–22), but no data have been presented to support or refute this hypothesis. Here we show, from studies in both the field and the laboratory, that both conditions for Crozier's paradox are satisfied in the invasive Argentine ant (Linepithema humile): rejected individuals incur a cost, and aggression is polarized with respect to genetic diversity. These findings suggest a mechanism to explain the widespread cooperation that characterizes introduced populations of this damaging invasive species.
The Argentine Ant
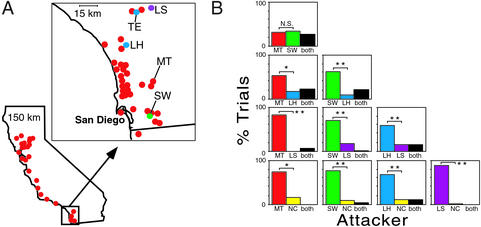
The colony structure of Argentine ants differs dramatically between the native and introduced ranges. In their native Argentina, L. humile populations consist of colonies that are typically tens to hundreds of meters in diameter (23, 24). These colonies contain multiple nesting sites (nests), each of which may contain workers, queens, and brood, and each colony's territory is aggressively defended against other Argentine ant colonies (20, 23–25). Native populations occur at low densities (relative to introduced populations) and coexist with other ants in species-rich communities (25). In contrast, introduced populations are typically unicolonial (26), forming spatially vast and competitively dominant supercolonies that lack territorial boundaries (20, 22–25, 27). For example, virtually all Argentine ants in California belong to the same supercolony (Fig. 1A, red circles), and workers, queens, and brood can be transferred freely among nest sites throughout the supercolony (20, 23, 24, 27). The resulting decline in aggression and intraspecific competition likely allows introduced Argentine ant populations to achieve high densities (28) and displace many species of native ants (29–32). Rarely, smaller supercolonies can be found in the introduced range (20, 22–25). In California, these colonies are spatially restricted, behave aggressively toward each other and the large supercolony (20, 23–25), and are genetically distinct from each other and the large supercolony (20, 23, 25). In both the native and introduced ranges, the rate of gene flow within colonies appears to be significantly higher than gene flow across colony boundaries. For example, nests within the large supercolony that are separated by >800 km are more genetically similar to each other than are neighboring nests that belong to different behaviorally defined colonies (20, 23, 24). This pattern likely arises because queens do not undergo a nuptial flight, but instead, mate within their natal colony (27). New nests arise via colony budding, when groups of queens and workers disperse on foot and establish a nest nearby (27).
Figure 1.
(A) The geographic distribution of supercolonies and sampling sites in California. Populations of Argentine ants identified as members of the large supercolony that dominates most of the introduced range are shown in red [sampled for this study at sites labeled MT and LJ (La Jolla)]. Three smaller supercolonies [LH/TE (LH/Temecula), SW, and LS] were sampled in southern California (Inset) and are shown in different colors. Site NC (Winston-Salem, NC) is not shown. (B) The frequency of aggression between workers from all pairwise comparisons of the five field colonies (n = 607 worker pairs). Aggressive rejection was polarized in nine of the 10 colony pairs (χ2; *, P < 0.01; **, P < 0.001; corrected for multiple comparisons; ref. 37). The comparison of MT and SW was not significantly polarized. The frequency of mutual aggression (both ants attack simultaneously) is indicated for each colony pair by the black bars.
Genetic and behavioral studies of Argentine ants in their native and introduced ranges suggest that a genetic bottleneck during introduction reduced levels of genetic diversity overall and at recognition (label) loci, initiating the transition to unicoloniality in introduced populations (20, 23, 24). This bottleneck increased overall levels of genetic similarity (20, 23, 24) and within-colony relatedness relative to the native range (20, 21, 24). Because Argentine ants possess a system for nestmate recognition that evolved in the genetically diverse native range, the increased similarity in introduced populations appears to promote widespread cooperative behavior (21, 23, 24). If the mechanism underlying Crozier's paradox also operates in introduced populations of the Argentine ant, it would reduce the potential for the establishment of colonies carrying novel recognition alleles, stabilize the unicolonial colony structure of introduced populations, and enhance the competitive ability of the supercolony (20, 21).
The Structure of Recognition Systems
When one individual (the actor) encounters another (the recipient), several components of the recognition system interact to produce acceptance or rejection (4, 7–13). First, an individual must carry a phenotypic label (or cue) that provides information about its identity. These labels may be genetically based, environmentally based, or some mixture of both (7, 8, 12). Argentine ants appear to use genetic labels, such as heritable odor cues, to identify an individual's group membership (22, 23, 33). Second, the actor must possess a sensory template against which to compare the label of the recipient. The template is a suite of labels that the actor accepts as self. The labels in an actor's template are based on a referent: an object, individual, or group of individuals that possesses the acceptable labels and that is used as a reference for formation of the template. In theory, referents can be self (in which the actor forms a template that includes only its own labels), relatives, other individuals in the social group, or a combination of these. In species that possess multiple genetic lineages per colony (as in Argentine ant colonies), workers typically learn or imprint on the labels (odor cues) present in the colony, thereby forming a template that is shared among colony members (7, 10). The stringency of a recognition system is thus a function of the breadth of the referent and the template that it forms; when the referent includes many individuals, a broader template is formed and more labels (and hence, individuals) are deemed acceptable. Acceptance occurs when the recipient's label matches the actor's template (a correct “phenotype match,” ref. 12). When the actor's template and the recipient's label do not match, the actor rejects the recipient as foreign. Argentine ants appear to possess a recognition system in which foreign labels are rejected, rather than one in which shared labels are accepted because intransitivities among colonies are extremely rare (ref. 14, see Results).
Methods
Behavioral Assay.
We used a standard behavioral assay in which we paired two individual workers together in a neutral arena for 5 min (25, 28). We scored interactions between the workers on a scale from 1 to 4: 1 = touch (physical contact, but no aggressive response; may include antennation or trophallaxis), 2 = avoid (the ants touch, and one or both recoils and runs in the opposite direction), 3 = aggression (a physical attack by one or both of the workers, including lunging, biting, or pulling of legs or antennae), and 4 = fighting (prolonged aggression, including prolonged biting and pulling and the use of chemical defensive compounds). We interpreted the initiation of aggression by one of the two workers as rejection. We used a combination of methods to distinguish workers from the two colonies during the trials including videotaping the trial for later analysis, and marking one of the ants by feeding it a small amount of dilute sugar water. There was no effect of marking on the behavioral outcome (χ2, P > 0.4). A previous analysis showed that this behavioral assay has a high repeatability and a low variance among trials within the same nest pairing (23). Although the behavioral assays were not initially performed by an observer who was blind to the identity of the ants used, we confirmed that no observer bias was introduced by having a naïve observer blindly rescore the videotaped trials (n = 31). A comparison of these scores with the original scores showed that only one attacker was misidentified, and this misidentification occurred opposite to the potential direction of bias. A correct identification of this attacker would have increased the significance of our results.
Microsatellite Genotyping.
We purified DNA from individual Argentine ant workers and genotyped each individual at 12 polymorphic microsatellite loci (GenBank accession nos. AF0983514, AF093517, AF093515, AF093520, AF093521, AF093522, AF093525, AF093526, AF093527, AF093531, AF093533, and AF173164; refs. 20, 25, and 34). We separated and visualized PCR products on an Applied Biosystems Prism 377XL automated sequencer and analyzed the gel files by using the program strand (35). We calculated the number of alleles, number of polymorphic loci, and expected heterozygosity (Hexp) using the program genalex (36).
Aggression Among Field-Collected Colonies.
We collected Argentine ants from five populations in the field and conducted a total of 607 behavioral assays between all possible colony pairs (n = 10 pairs). We chose these populations because previous surveys had shown them to be aggressive toward each other (20, 23–25, 28). One of these, Mission Trails (MT), belongs to the large supercolony. The other three belonged to smaller colonies: Lake Hodges (LH), Lake Skinner (LS), and Sweetwater Reservoir (SW). Argentine ants were also collected from a fifth population in Winston-Salem, NC.
When aggression occurred between workers, we noted the individual that initiated aggression (the aggressor) and the individual that was the victim of the initial attack (the recipient). We performed at least 30 assays between each colony pair (mean number of trials per colony pair = 60.7 ± 5.3 SE). No worker was used in more than one assay. We preserved workers from 310 of the 607 behavioral assays (n = 610 workers; 58–68 workers per colony pair) for genotyping at 12 polymorphic microsatellite loci. Although estimates of genetic diversity using the number of alleles or the number of polymorphic loci may be sensitive to differences in sample size, we avoided this problem by not combining data across different colony pairs (see below for additional details). Consequently, for each colony in a colony pair, the number of workers sampled is equal.
To avoid biases associated with genetic changes within colonies through time we did not combine genetic data across colony pairings. For example, ants collected from MT in March 2002 possessed different alleles and alleles at different frequencies than samples collected previously from the same site. Moreover, the results of previous studies show that field colonies in the introduced range can exhibit changes in behavior and cuticular hydrocarbons through time (although colonies maintained in the laboratory for the same time period did not exhibit such changes; ref. 33).
Survival of Attacker vs. Recipients of Aggression.
We scored the survival of attackers versus recipients in 281 of the 607 behavioral assays. To do this, we conducted behavioral assays as above, but permitted the trial to continue for 2 h if aggression occurred. We examined the individuals every 2–4 min and noted their physical condition. After 2 h, we recorded the number and identity of surviving workers.
Low-Diversity, Single-Queen Subcolonies.
To examine the relationship between relative levels of genetic diversity and polarized aggression we manipulated genetic diversity in a laboratory experiment. We collected Argentine ant workers and queens from four source colonies in California and used them to generate 28 subcolonies. The collection sites were SW (used to construct five subcolonies), LH (eight subcolonies), Temecula (TE; six subcolonies), and La Jolla (LJ; seven subcolonies). The nest from LJ belongs to the large supercolony whereas the nests collected at SW, LH, and TE are members of smaller, behaviorally different colonies (Fig. 1A). We initiated each experimental subcolony with a single dealate (mated) queen and 20 workers and maintained them in a 125 × 50-mm Fluon-coated Petri dish. Because Argentine ant colonies often contain dozens of reproductive queens per square meter (27), the original 20 workers were likely produced by many different queens. We fed the colonies dead crickets or scrambled eggs every 3 days and provided continuous access to sugar water. Several previous studies have shown that larger colony fragments (that contain multiple queens and large numbers of workers) do not exhibit behavioral changes when maintained in the laboratory for periods up to or exceeding 1 year (22, 33).
We maintained the subcolonies in the laboratory for 8 months, by which time daughters of the single queen would have replaced the initial 20 workers. We therefore expected that the level of genetic diversity within the subcolonies would decrease through time (this prediction was later confirmed by microsatellite genotyping). Relative to workers from the original source colonies, workers that develop in this low-diversity environment should possess fewer labels, develop a narrower, more stringent template, and initiate aggression when paired against workers from the original source colonies. Under normal circumstances (in the field), the workers that were paired against each other in the behavioral assays would have been colony mates because their mothers were former colony mates in the field.
After 8 months, we conducted behavioral assays between workers from these low-diversity colonies and workers from their original source colony. First, we collected large numbers of ants (>50 queens, >10,000 workers) from the original source locations, separated the ants from soil and nesting material, and maintained them in the laboratory for 2–8 weeks. We then conducted behavioral assays between these workers and the workers produced in the low-diversity, single-queen subcolonies (n = 124 behavioral assays; mean no. trials per colony pair = 4.8 ± 0.1 SE). After the behavioral assays, we genotyped all individual workers at 12 polymorphic microsatellite loci. One worker from one source colony (SW) was triploid at two loci, suggesting diploid male production. This individual was excluded from subsequent analyses.
Results
Aggression Among Field-Collected Colonies.
Aggression between colonies was typically polarized, with workers from one of the two colonies acting as aggressors significantly more often (Fig. 1B; P < 0.05 in nine of the 10 colony pairs, χ2 corrected for multiple comparisons; ref. 37). The colonies also formed a behavioral hierarchy, with workers from the large supercolony (MT) and one of the smaller supercolonies (SW) acting as aggressors in pairings with all other colonies.
Genetic analysis using microsatellite markers showed that workers from less genetically diverse colonies were more often the aggressors. In all but two of the colony pairs, the colony that initiated aggression more often possessed fewer alleles overall (Fig. 2B; P = 0.0547, sign test), and in all cases the colony that initiated aggression more often possessed fewer polymorphic loci (Fig. 2A; P < 0.001, sign test). This pattern was not significant, however, when expected heterozygosity was used as the measure of genetic diversity (Fig. 2C; P = 0.377, sign test). In all cases, MT and SW possessed lower levels of genetic diversity (by all three measures) than the colonies they were paired against.
Figure 2.
Genetic diversity in colonies that acted as the attacking colony (x axis) relative to the levels of genetic diversity in colonies that were the recipients of aggression (y axis). The diagonal line represents equal levels of genetic diversity between colonies; points above this line represent colony pairs in which the more aggressive colony possessed lower levels of genetic diversity than the recipient colony. The nonpolarized colony pair (MT vs. SW) is shown in gray. Colonies that acted as attackers typically possessed fewer alleles than the colonies that they attacked (sign test, P = 0.0547) (A) and always possessed the same number or fewer polymorphic loci (sign test, P < 0.001) (B), but did not have lower expected heterozygosity (Hexp) (C). The exceptions (below the diagonal) were always pairings of two high-diversity colonies. The number of workers genotyped from each colony is exactly equal for each individual data point. Superimposed points are offset for clarity.
Survival of Attacker vs. Recipients of Aggression.
We could unambiguously identify an aggressor in 193 of the 281 assays conducted to assess survival (there was no aggression in 52 trials; in 36 trials both workers simultaneously displayed aggression). In these trials, there were three types of outcomes: both workers died (22.7% of the trials), both workers lived (18.2%), or one worker survived (59.1%). When only one of the two workers survived, the aggressors survived six times more often than recipients (Fig. 3; P < 0.0001, χ2). This difference in survival appears to be a result of aggressive behavior rather than intercolony differences in fighting ability. In the rare cases when the aggressor was from a colony that was typically the recipient of aggression, the aggressor still survived significantly more often than the recipients and by a similar margin. Specifically, in 58 of the trials, the attacking worker was from a colony that was typically the recipient of aggression. Only 15 of these trials ended in the death of only one of the workers; in 13 of these the attacker was the survivor (P < 0.01, χ2).
Figure 3.
Survival of Argentine ant workers 2 h after an aggressive encounter (n = 281 trials). Workers that initiated aggression (Aggressor) survived significantly more often than the recipients of aggression (χ2, P < 0.0001).
Low-Diversity, Single-Queen Subcolonies.
The worker population in the single-queen subcolonies possessed significantly lower levels of genetic diversity than workers from the original source colonies in the field (Fig. 4). Behavioral assays between workers from the low-diversity, single-queen subcolonies and workers from their original source colony showed that 24 of the 26 colony pairs exhibited aggression. In six of the 24 aggressive colony pairs, workers from both colonies behaved aggressively in at least one assay. In the remaining 18 colony pairs aggression was consistently polarized, with workers from only one of the two colonies initiating aggression. As with interactions between workers from the field colonies, workers from the experimental, low-diversity subcolonies initiated aggression significantly more often than workers from the more diverse source colonies (Fig. 5; 14 of 18 polarized interactions between colonies; P < 0.05, χ2).
Figure 4.
Differences in genetic diversity and behavior between the single-queen subcolonies (Lab) and in the original source colonies used to initiate the single-queen subcolonies (Source). The single-queen subcolonies possessed fewer alleles overall (±95% confidence interval) (A), fewer polymorphic loci (±95% confidence interval) than their original source colonies (B), and significantly lower expected heterozygosity (Hexp; ±95% confidence interval) (C).
Figure 5.
Polarized aggression between workers from the single-queen subcolonies (LAB) and the source colony. Workers that developed in the low-diversity, single-queen subcolonies displayed higher levels of aggression toward workers from their original source colony (overall: χ2, P < 0.05).
Discussion
Our findings suggest that positive frequency-dependent selection is operating in introduced populations of the Argentine ant through the asymmetric rejection of individuals from high-diversity colonies. Combined with previous findings, the present study reveals how alterations in label diversity (and, as a result, template breadth; refs. 7–13) may have facilitated the Argentine ant's remarkable transition in social structure and promoted the maintenance of unicoloniality in the introduced range. The first steps in the origin of unicoloniality probably occurred during or soon after introduction. Features of the introduced range (such as the availability of disturbed habitat and the absence of coevolved predators, parasites, and competitors) likely facilitated the establishment and growth of introduced propagules. Additionally, the loss of genetic diversity after a population bottleneck (and subsequent genetic drift) would have increased the level of genetic similarity among individuals in the introduced range (both overall and at recognition loci), promoting the expression of cooperative or altruistic behaviors (20, 23, 24). The annual bottlenecks that occur each spring after Argentine ant workers execute large numbers of their queens (27) should reinforce the effects of this founder event (21). This loss of genetic diversity would have resulted in polarized aggression toward genetically different groups, as reported here. This pattern of aggression could also limit the establishment of new, genetically diverse introductions from the native range, as well as produce high levels of aggression toward novel genetic variants that arise in the introduced range. Although fusion among some populations that descended from separate introductions may have occurred, such events would have been limited to genetically similar populations. As supercolonies expanded in the introduced range, the numerical advantages associated with large colony size (28) probably increased, further strengthening the impact of directional aggression.
This scenario for the transition to unicoloniality in introduced Argentine ant populations fundamentally differs from previous explanations (22) in several respects. First, we recognize that a population bottleneck has reduced genetic diversity in introduced populations (20, 23, 24), which has, in turn, produced polarized aggression between colonies with respect to their relative levels of genetic diversity. This finding contrasts with previous assertions (22) that introduced populations experienced a weak (although statistically significant) loss of genetic diversity during introduction. The difference between Giraud et al. (22) and Tsutsui et al. (23) regarding the severity of the genetic bottleneck likely arises from the different sampling regimes used. Giraud et al. compared samples from 33 sites in Europe to Argentine ants from a single site in the native range (22) whereas Tsutsui et al. compared introduced Argentine ants from 17 sites in California (35 nests) to individuals from 12 sites (29 nests) in the native range (23).
The mechanism proposed here also differs from previous explanations (22) in that it does not invoke a relaxation of ecological constraints to explain the loss of genetic diversity at recognition loci. Although it is clear that introduced Argentine ant populations attain higher densities than native populations do, the resulting increased encounter rate between nests and colonies should lead directly to reductions in genetic diversity (overall and at recognition loci) via polarized aggression. This finding contrasts with the previous model (22), in which high population densities and increased encounter rates increase the costs of territory defense, thereby producing reduced intercolony aggression, regardless of the genetic similarity of the colonies.
Given the operation of frequency-dependent selection against rare recognition alleles, there are several reasons current levels of genetic variation at microsatellite loci (which we assume do not directly affect recognition) should also predict the polarity of agonistic behavior in these populations. The genetic bottleneck that accompanied the initial introduction would have equally reduced diversity across the genome, including both recognition and microsatellite loci. Subsequently, the rate at which variation at loci under selection becomes dissociated from variation at other loci depends on the strength of selection, selfing rate, and linkage between the two classes of loci (38). In particular, if numerous recognition loci were widely dispersed throughout the genome then it could take many generations for selection and recombination to break down the association between microsatellite and recognition loci. A critical first step toward distinguishing among the contributions of selection, breeding system, and linkage to this association will require the identification of recognition loci and direct examination of how positive frequency-dependent selection acts on them.
The results of the laboratory experiment have four important implications. First, a genetic bottleneck appears to lead to asymmetrical aggression between colonies, with the low-diversity colonies that passed through the bottleneck attacking high-diversity colonies. This finding supports the proposal that the genetic bottleneck that occurred during the Argentine ant's introduction could initiate a process of runaway positive frequency-dependent selection (20, 21). Second, intraspecific aggression is rare in introduced populations of Argentine ants (20–27). However, contrary to previous suggestions (22), the aggression displayed by the subsamples of field colonies indicates that the field colonies still retain some level of polymorphism at recognition loci. Third, long-term studies of larger, polygynous (multiple-queen) laboratory colonies showed that worker behavior remains virtually unchanged for >1 year (22, 33). Thus, the heightened aggression we observed in these experiments appears to be a consequence of the reduced queen number and corresponding reduction in genetic diversity, rather than an artifact of culture under laboratory conditions. Fourth, previous studies in other species demonstrated that the recognition abilities of workers from polygynous colonies are typically less stringent than those of workers from monogynous (single-queen) colonies (39–41). Our results support the hypothesis that differences in label diversity and template breadth account for the reduced discriminatory ability in polygynous colonies (39–41) and are consistent with theoretical studies of the evolution of optimal acceptance thresholds in recognition systems (11).
Although the observed asymmetries in aggression and mortality cannot affect the direct fitness of Argentine ant workers (which are completely sterile), the loss of large numbers of workers should compromise colony function. In Argentine ants, small colony size is associated with reductions in foraging activity, resource discovery rate, resource retrieval rate, resource defense ability (intraspecific and interspecific), brood production, worker production, interspecific competitive ability, and propagule survival (28, 32, 42, 43). It remains to be seen, however, if the observed asymmetries in aggression and mortality between individual workers translate directly into differences in performance or productivity at the level of entire colonies.
It is important to note that this selection requires direct interaction between conspecific colonies. If colonies are patchily distributed or isolated, then the frequency of intercolony interactions will be low and the costs of aggressive rejections should rarely be exacted. Additionally, Argentine ant colonies in their native range combine high levels of genetic diversity with high levels of genetic differentiation over relatively small spatial scales (24). Under these circumstances, aggression should be mutually expressed, rather than polarized, and frequent. Heightened parasitism, predation, or interspecific competition relative to that experienced by introduced populations may further prevent native populations from achieving high population densities or large colony size, thereby limiting the frequency of intraspecific, intercolony interactions.
The expansive unicoloniality displayed by introduced populations of Argentine ants may be evolutionarily unstable because workers display altruistic behavior toward individuals to which they may be only distantly related (44). Under these circumstances, the heritability of adaptive and maladaptive worker traits decreases, and kin selection cannot operate efficiently (44). The rarity of unicoloniality in ants generally is consistent with this prediction (5, 13). Additionally, as genetic diversity declines in introduced populations, other maladaptive traits may arise, such as increased frequency of diploid males or heightened susceptibility to pathogens or parasites.
Despite the rarity of unicoloniality generally, many of the most widespread and damaging invasive ants are unicolonial (45). As in Argentine ants, the combined impacts of genetic bottlenecks and runaway positive frequency-dependent selection may account for the transition in colony structure between native and introduced populations. Currently, little is known about the biology of many of these invaders, particularly in native populations (45). Future work that compares behavior, colony structure, and population genetics of such species in both their native and introduced ranges will reveal if they achieved unicoloniality through processes similar to those experienced by the Argentine ant.
Acknowledgments
We thank T. J. Case, J. H. Gillespie, M. E. Hauber, D. A. Holway, P. S. Ward, A. C. C. Wilson, and two anonymous reviewers for valuable comments and criticism; J. Silverman and G. Buzcowski for providing Argentine ants from North Carolina; S. L. Bernauer, K. G. Cloud, S. C. Ostrem, and R. J. Toonen for technical assistance; and B. T. Anderson for assistance with the laboratory ant colonies. This work was supported by National Science Foundation Grant OCE 99-06741, the Miller Institute for Basic Research in Science, and the United States Department of Agriculture National Research Initiative Competitive Grants Program (Grant 00-35302-9417).
Abbreviations
- MT
Mission Trails
- LH
Lake Hodges
- LS
Lake Skinner
- SW
Sweetwater Reservoir
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Maynard Smith J, Szathmáry E. The Major Transitions in Evolution. Oxford: Freeman; 1995. [Google Scholar]
- 2.Dugatkin L A. Cooperation Among Animals: An Evolutionary Perspective. New York: Oxford Univ. Press; 1997. [Google Scholar]
- 3.Hamilton W D. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 4.Crozier R H, Pamilo P. Evolution of Social Insect Colonies: Sex Allocation and Kin Selection. Oxford: Oxford Univ. Press; 1996. [Google Scholar]
- 5.Bourke A F G, Franks N R. Social Evolution in Ants. Princeton: Princeton Univ. Press; 1995. [Google Scholar]
- 6.Wilson E O. In: Kin Recognition in Animals. Fletcher D J C, Michener C D, editors. New York: Wiley; 1987. pp. 7–18. [Google Scholar]
- 7.Sherman P W, Reeve H K, Pfennig D W. In: Behavioral Ecology: An Evolutionary Approach. Krebs J R, Davies N B, editors. Oxford: Blackwell; 1997. pp. 69–96. [Google Scholar]
- 8.Crozier R H, Dix M W. Behav Ecol Sociobiol. 1979;4:217–224. [Google Scholar]
- 9.Ratnieks F L W. Am Nat. 1991;137:202–226. [Google Scholar]
- 10.Breed M D, Bennett J M. In: Kin Recognition in Animals. Fletcher D J C, Michener C D, editors. New York: Wiley; 1987. pp. 243–285. [Google Scholar]
- 11.Reeve H K. Am Nat. 1989;133:407–435. [Google Scholar]
- 12.Lacy R C, Sherman P W. Am Nat. 1983;121:489–512. [Google Scholar]
- 13.Hölldobler B, Wilson E O. The Ants. Cambridge, MA: Harvard Univ. Press; 1990. [Google Scholar]
- 14.Grosberg R K. Q Rev Biol. 1988;63:377–412. [Google Scholar]
- 15.Richman A D. Mol Ecol. 2000;9:1953–1963. doi: 10.1046/j.1365-294x.2000.01125.x. [DOI] [PubMed] [Google Scholar]
- 16.Crozier R H. Evolution. 1986;40:1100–1101. doi: 10.1111/j.1558-5646.1986.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 17.Crozier R H. In: Kin Recognition in Animals. Fletcher D J C, Michener C D, editors. New York: Wiley; 1987. pp. 55–73. [Google Scholar]
- 18.Crozier R H. In: Invertebrate Historecognition. Grosberg R K, Hedgecock D, Nelson K, editors. New York,: Plenum; 1988. pp. 143–156. [Google Scholar]
- 19.Elgar M A, Crozier R H. Trends Ecol Evol. 1989;4:288–289. [Google Scholar]
- 20.Tsutsui N D. Ph.D. thesis. San Diego: University of California; 2000. [Google Scholar]
- 21. Tsutsui, N. D. & Suarez, A. V. (2003) Conserv. Biol., in press.
- 22.Giraud T, Pedersen J S, Keller L. Proc Natl Acad Sci USA. 2002;99:6075–6079. doi: 10.1073/pnas.092694199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsutsui N D, Suarez A V, Holway D A, Case T J. Proc Natl Acad Sci USA. 2000;97:5948–5953. doi: 10.1073/pnas.100110397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsutsui N D, Case T J. Evolution. 2001;55:976–985. doi: 10.1554/0014-3820(2001)055[0976:pgacso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Suarez A V, Tsutsui N D, Holway D A, Case T J. Biol Invasions. 1999;1:43–53. [Google Scholar]
- 26.Hölldobler B, Wilson E O. Naturwissenschaften. 1977;64:8–15. [Google Scholar]
- 27.Markin G P. Ann Entomol Soc Am. 1970;63:1238–1242. [Google Scholar]
- 28.Holway D A, Suarez A V, Case T J. Science. 1998;282:949–952. doi: 10.1126/science.282.5390.949. [DOI] [PubMed] [Google Scholar]
- 29.Ward P S. Hilgardia. 1987;55:1–16. [Google Scholar]
- 30.Human K, Gordon D M. Oecologia. 1996;105:405–412. doi: 10.1007/BF00328744. [DOI] [PubMed] [Google Scholar]
- 31.Suarez A V, Bolger D T, Case T J. Ecology. 1998;79:2041–2056. [Google Scholar]
- 32.Holway D A. Ecology. 1999;80:238–251. [Google Scholar]
- 33.Suarez A V, Holway D A, Liang D, Tsutsui N D, Case T J. Anim Behav. 2002;64:697–708. [Google Scholar]
- 34.Krieger M J B, Keller L. Mol Ecol. 1999;8:1075–1092. [Google Scholar]
- 35.Toonen R J, Hughes S. BioTechniques. 2001;31:1320–1324. [PubMed] [Google Scholar]
- 36.Peakall R, Smouse P E. GenAlEx V5: Genetic Analysis in Excel. Canberra: Australian National University; 2001. [Google Scholar]
- 37.Rice W R. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 38.Hedrick P W. Genetics of Populations. Sudbury, MA: Jones and Barlett; 2000. [Google Scholar]
- 39.Starks P T, Watson R E, Dipaola M J, Dipaola C P. Ethology. 1998;104:573–584. [Google Scholar]
- 40.Vander Meer R K, Obin M S, Morel L. In: Applied Myrmecology: A World Perspective. Vander Meer R K, Jaffe K, Cedeno A, editors. Boulder, CO: Westview; 1990. pp. 95–101. [Google Scholar]
- 41.Pirk C W W, Neumann P, Moritz R F A, Pamilo P. Behav Ecol Sociobiol. 2001;49:366–374. [Google Scholar]
- 42.Hee J J, Holway D A, Suarez A V, Case T J. Conserv Biol. 2000;14:559–563. [Google Scholar]
- 43.Holway D A, Case T J. Anim Behav. 2001;61:1181–1192. doi: 10.1006/anbe.1999.1329. [DOI] [PubMed] [Google Scholar]
- 44.Queller D C, Strassmann J E. BioScience. 1998;48:165–175. [Google Scholar]
- 45.Holway D A, Lach L J, Suarez A V, Tsutsui N D, Case T J. Annu Rev Ecol Syst. 2002;33:181–233. [Google Scholar]