Abstract
The evolutionary transition from single cells toward multicellular forms of life represents one of the major transitions in the evolution of complex organisms. In this transition, single autonomously reproducing cells became parts of larger reproducing entities that eventually constituted a new unit of selection. The first step in the evolutionary transition to multicellularity likely was the evolution of simple, undifferentiated cell clusters. However, what the selective advantage of such cell clusters may have been remains unclear. Here, we argue that in populations of unicellular organisms with cooperative behavior, clustering may be beneficial by reducing interactions with noncooperative individuals. In support of this hypothesis, we present a set of computer simulations showing that clustering can evolve as a biological, heritable trait for cells that cooperate in the use of external energy resources. Following the evolution of simple cell clusters, further benefits could have arisen from the exchange of resources between cells of a cluster.
The main benefits of multicellularity arise from the division of labor between differentiated cells (1). Accordingly, many studies on the evolution of multicellularity have focused on the advantages of differentiation (1, 2) and of separation of soma and germ-line cells (3, 4). However, it is unlikely that these advantages have been the driving force in the evolutionary transition, because the evolution of differentiated multicellular organisms was presumably based on multicellular ancestors with no or little differentiation. Such simple undifferentiated organisms could have easily evolved, for example, by a mutation that prevents dividing cells from complete separation (5–7). Although it is easy to see how simple cell clusters could have emerged, it is unclear what the selective advantage of clustering cells over their unicellular ancestors could have been. Size has been suggested as an important factor in the evolution of multicellularity (6, 8, 9), but the precise nature of the selective advantage of size remains unclear. On the other hand, there are likely disadvantages associated with the clustering of cells. Compared with their unicellular ancestors, clustering cells face locally increased cell densities. Thus, competition for local resources is increased. Furthermore, a mutation leading to clustering would likely hamper the mobility of organisms that rely on passive or active motion in the search for food resources. What factor, then, could have driven the evolution of early, undifferentiated stages in the transition to multicellularity?
Here, we examine whether clustering may evolve in populations of unicellular organisms with cooperative behavior if it allows cooperating individuals to reduce interactions with noncooperative competitors. Examples of cooperative behavior among unicellular organisms are cooperative hunting, the excretion of exo-enzymes for feeding on external resources, or the production of anti-competitor toxins (8, 10–12). Here, we consider a form of cooperation in the feeding on external energy resources, which is based on a tradeoff between rate and yield in heterotrophic ATP production (13). Efficient ATP production with high yield (units of ATP per unit of resource) rather than high rate (units of ATP per unit of time) can be seen as a form of cooperation, because in a population of efficient resource users, the benefit in terms of ATP is high for all individuals. On the other hand, fast but inefficient ATP production is noncooperative because the benefits resulting from the high rate of ATP production are confined to the individual, whereas the costs of inefficient resource use are shared among all users of the resource. Efficient resource use represents a very general form of cooperation, because tradeoffs between rate and yield in ATP production, and probably also in the use of ATP in biosynthetic pathways (14), are for fundamental thermodynamic reasons present in all heterotrophic organisms (15, 16).
By using computer simulations (13) and analytical approaches (17), we have shown previously that cooperation in the use of external energy resources may evolve in spatially structured environments. A large body of theory suggests that the evolution of cooperation is generally facilitated by spatial structure because of the increased frequency of interaction among relatives or clones in an asexual population (18). However, it has been argued more recently that increased interactions also lead to increased competition among relatives, which in turn may erode the benefits of cooperation (19–22). Cooperation in the use of external energy resources can evolve in a spatially structured environment because some of the benefits of efficient resource use flow back to the individual, particularly if interacting with only few competitors (17).
Methods and Results
For our simulations, we assume that cells live in an aerobic environment and feed on energy resources such as carbohydrates that can be metabolized by fermentation and respiration. The simulations take place on a square grid with periodic boundary conditions. At each grid site, there is an amount of resource and maximally one cell. A cell consumes resources at its site to produce ATP. We assume that there are two metabolic types of cells with opposing properties in ATP production. The first type, called a respirator, uses respiration exclusively, and thus converts the energy resource with high efficiency. In line with biochemical observations (23–25), we assume that ATP production by respiration is restricted to a low rate. The second type, called respiro-fermentor, uses fermentation in addition to respiration and, therefore, produces ATP faster but with lower total efficiency. Such respiro-fermentative metabolism is a typical mode of ATP production in unicellular eukaryotes such as yeasts (23–25). The rates of resource consumption and ATP production are assumed to be saturating functions of the resource level and are given in Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org. Once a cell has synthesized a sufficient amount of ATP and at least one of the four directly neighboring sites is empty, it can divide, and the daughter cell is placed on one of the free neighboring sites. We assume that a cell produces ATP only when necessary. Thus, if a cell has synthesized enough ATP to divide but cannot divide because there is no empty site in the neighborhood, it stops resource consumption and ATP production. Death of cells occurs with a fixed probability per time unit. We implement cell motion by swapping cells on randomly chosen neighboring grid sites. Finally, resource is added stochastically to grid sites and diffuses to neighboring sites. Further details are given in Supporting Text. Time courses and snapshots are shown in Fig. 2, which is published as supporting information on the PNAS web site.
Simulation 1.
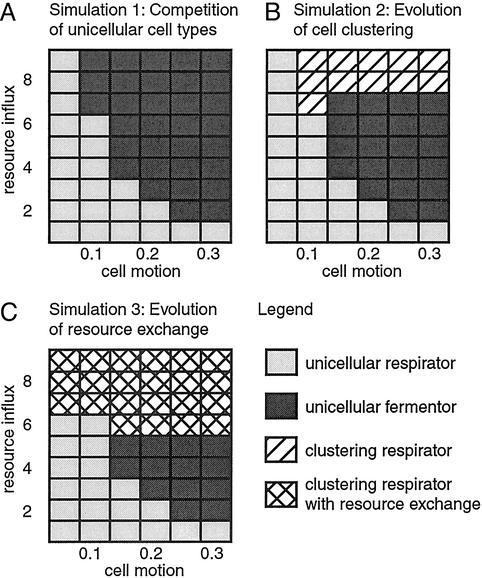
The basic model of spatial resource competition presented above describes the competition between “unicellular” respiro-fermentors and respirators, but does not yet contain any features of multicellularity. The results of the simulation of the basic model (Simulation 1) for different rates of cell motion and resource influx are shown in Fig. 1A. The simulations show that unicellular respirators prevail over unicellular respiro-fermentors at low rates of cell motion and resource influx. This result is in agreement with previous results of ours (13). High resource influx generally results in higher cell densities, which in turn leads to increased competition for local resources. Hence, high rates of resource influx are favorable for fast resource users, whereas efficient resource users can persist at low resource influx. Low rates of cell motion are favorable for efficient resource users because cells of the same metabolic type tend to form loose aggregates, and hence, respirators may benefit from their efficient resource use. With increasing motion of the cells, the different metabolic types become more mixed, and hence, fast but inefficient resource use is favored. Note that cooperation in the use of external energy resources is not based on direct interactions between individuals but is mediated indirectly through the environment. That is, the strategy of an individual directly affects its environment. Thus, if an individual is alone or interacts only with few competitors, as is the case at low cell densities and at low rates of cell motion, the benefits of efficient resource use flow back to the individual.
Figure 1.
Spatial resource competition between respirators and respiro-fermentors and the evolution of cell clustering and resource exchange. (A) The results of competition between the unicellular cell types (Simulation 1) show that respirators outcompete respiro-fermentors at low rates of cell motion and low resource influx. (B) The simulation of competition between unicellular and clustering cell types (Simulation 2) shows that clustering respirators may evolve at high resource influx rates. Clustering respirators occupy a parameter range that was confined to unicellular respiro-fermentors in Simulation 1. (C) Simulation 3 shows that, after the evolution of cell clusters, resource exchange between cells of a cluster provides a further selective advantage. The parameter range that was occupied by simple clustering respirators in Simulation 2 is dominated by resource-exchanging respirators.
Simulation 1 shows that if an external factor such as low cell motion leads to the aggregation of unicellular organisms of the same metabolic type, then cooperative, efficient resource users have a selective advantage over noncooperative, fast resource users. Thus, the simulation suggests that aggregation drives the evolution of cooperative resource use. However, we have not yet shown that cooperative resource use could drive the evolution of cell clusters, where cells actively stick to each other. The spatial aggregation of cells in Simulation 1 is not an intrinsic biological, heritable property of the cells but rather a property of the environment, because both cell types move independently and at an externally determined rate. For the evolution of multicellularity, one needs to show that cell clustering can evolve as a heritable trait.
Simulation 2.
To address this point, we introduce two further cell types that actively form cell clusters (“clustering respirators” and “clustering respiro-fermentors”). In contrast to the unicellular cell types, clustering implies that the daughter cell always remains attached to the mother cell and cannot diffuse apart. Because a mutation leading to such clustering would likely reduce cell motility or diffusion, we assume, for simplicity, that cells of clustered types are immobile. Otherwise, the two clustering cell types have the same properties as the corresponding unicellular cell types. Control simulations (not shown) of pair-wise competition between clustering and unicellular respiro-fermentors and between clustering and unicellular respirators, respectively, show that the mobile, unicellular cell type always outcompetes the clustering type. Thus, clustering per se is disadvantageous. This observation is in agreement with theoretical studies about the evolution of dispersal rates, which have shown that dispersal is often favored because it reduces competition among relatives (26–28).
To determine whether clustering is advantageous in competition with noncooperative, unicellular organisms, we ran simulations with all four cell types present simultaneously (Simulation 2). The results, shown in Fig. 1B, demonstrate the following points. First, at high levels of resource influx, clustering respirators prevail in competition over the other cell types. In Simulation 1, this parameter domain was occupied by unicellular respiro-fermentors. The dynamics in this parameter range resemble a rock-scissors-paper game, because in pair-wise competition, unicellular respirators can outcompete clustering respirators (control simulations), unicellular respiro-fermentors can replace unicellular respirators (Simulation 1), and clustering respirators can invade into a population of unicellular respiro-fermentors (Simulation 2). In the simulation, cycling between these three types is observed at a local scale. On average, clustering respirators persist at the highest level. Both other types can temporarily dominate small patches on the grid but are, on average, present at lower levels. Note that the evolution of clustering respirators from unicellular respiro-fermentors requires either the simultaneous change of two properties, or maybe more realistically, a heterogeneous environment with patches that differ in their characteristics. In such an environment, both properties could have evolved in succession.
Simulation 2 shows that clustering can be a selective advantage for efficient resource users when in competition with inefficient ones. By clustering, respirators can shield their resource against the faster resource-consuming respiro-fermentors and reap the full benefits of efficient resource conversion. Such shielding is possible because we explicitly assume that there is competition for energy resources and for space (i.e., there is maximally one cell per grid site). Thus, clustering is advantageous here because it excludes selfish individuals from clusters of cooperators. Because clustering results from imperfect cell division, it provides a mechanism to interact preferentially with clones with the same strategy in resource use. Therefore, clustering is only beneficial for efficient resource users but not for inefficient ones. In our simulation, the advantage of clustering must compensate for intrinsic disadvantages that are associated with clustering (see control simulations). First, clustering cells have high local cell densities. Thus, they exhaust local resources faster than their unicellular competitors. Furthermore, cells internal to the cluster cannot divide even if they have synthesized a sufficient amount of ATP, because they have no free sites in their neighborhood. Second, as mentioned above, immobility, which in our model is inextricably linked to clustering, is disadvantageous. These disadvantages are less pronounced if the competing unicellular population is also present at high cell densities, as is the case at high rates of resource influx. Here, the advantages of clustering outweigh the disadvantages. However, if the competing population of unicellular individuals is present at low density, as is the case at a low rate of resource influx, the disadvantages of clustering become more pronounced and clustering cannot evolve.
The clustering cell types share a fundamental characteristic with multicellular organisms, in that the advantage of clustering arises from the properties of the entire cluster rather than the individual cell. In that sense, the unit of selection is shifted from the individual to the cluster. Importantly, this shift is a consequence of a heritable, biological property. Observations in myxobacteria suggest a link between cooperation and clustering. Myxococcus xanthus displays cooperative behavior in feeding and dispersal (10, 11). Cheater mutants have been identified that, measured over the entire life cycle, achieve an overrepresentation in the fruiting body at the expense of wild-type strains (29, 30). In line with our simulations, wild-type strains adhere to each other during cooperative feeding, whereas the cheater strains have lost their adhesion (ref. 31; Greg Velicer, personal communication).
Simulation 3.
A possible step after the evolution of simple cell clustering could have been the exchange of energy resources between cells belonging to the same cluster, which may result, for example, from a mutation that leads to imperfect separation of the cytosol after cell division (7). Resource exchange may be advantageous because it allows shuttling of resources from high resource sites, where respiration is saturated, to low resource sites, where it is not. In this way, resource may be used for ATP production not only at sites with high resource levels but in all cells of a cluster. Such “parallel processing” of energy resources would allow a cell cluster of efficient resource users to obtain a higher total rate of ATP production. To test whether the exchange of energy resources is indeed beneficial, we include two further clustering cell types in our model: “resource-exchanging respiro-fermentors” and “resource-exchanging respirators.” For cells of these cell types, we assume that the exchange of energy resources between cells of a cluster is driven by diffusion. All other properties of the resource-exchanging cell types are identical to the clustering cell types without resource exchange. Fig. 1C shows the simulation results of competition of all six cell types (Simulation 3). The results show that resource-exchanging respirators generally perform better than clustering respirators without resource exchange. The entire parameter domain that was occupied by clustering respirators without resource exchange in Simulation 2 (Fig. 1B) is now dominated by resource-exchanging respirators. Furthermore, resource-exchanging respirators can occupy parts of the parameter space previously occupied by unicellular respirators and unicellular respiro-fermentors. Thus, Simulation 3 shows that a mutation leading to resource exchange could be beneficial for clustering cells. Note that this benefit does not require cell differentiation.
The exchange of metabolites is a common characteristic in simple and complex multicellular organisms. The lack of crosswalls in many mycelial heterotrophs and their capability of outgrowth into substrate-deficient media indicate active or passive translocation of metabolites (32, 33). Furthermore, the accumulation of glycogen in different parts of mycelial actinomycetes supports the translocation particularly of energy resources in these prokaryotes (34). Experimental studies based on [14C]-labeled glucose analogues provide direct evidence for the transport of energy resources in mycelial fungi (33, 35). Thus, resource exchange could have been a key step in the evolutionary transition to multicellularity enhancing the benefits of clustering. The resources of individual cells are then integrated into a resource pool of the entire cluster. In this way, the reproduction of individual cells becomes linked with the success of the entire cluster, thereby completing the shift in the unit of selection from the individual to the cluster.
Discussion
Multicellularity evolved several times independently in a number of different taxa (5, 6). Hence, it is unlikely that there is a single explanation for the origin of multicellularity. Our simulations show that cooperation in the use of external energy resources provides one mechanism to drive the evolution of simple undifferentiated cell clusters. This mechanism applies to heterotrophs but not to phototrophs. In principle, other types of cooperative behavior that do not require cell specialization, such as exo-enzyme excretion or toxin production, could have driven the evolution of undifferentiated cell clusters in an analogous way. Note that these types of cooperative behavior can emerge easily in unicellular populations because they are mediated through the environment and may lead to direct benefits for cooperators. Furthermore, environmentally mediated behavior allows the evolution of condition-dependent behavior if individuals can recognize the state of the environment. Condition-dependent dispersal, for example, may lead to assortment processes that, in turn, facilitate the evolution of cooperative traits (36).
The selective advantage for clustering of cooperating organisms results from reduced interactions with noncooperating individuals. This advantage arises from the properties of the cluster rather than the individual and, thus, may represent the first step in the transition to multicellularity. However, undifferentiated cell clusters still lack other important features of multicellular organisms. In particular, cells of such a cluster still reproduce independently. A next step leading to the evolution of linked reproduction could have been the exchange of resource between cells belonging to the same cluster. Like cooperative resource use, resource exchange provides a selective advantage that does not require cell differentiation. Following the evolution of cell clusters and of metabolite exchange, the path is open for the evolution of cell differentiation.
Supplementary Material
Acknowledgments
We thank Martin Ackermann, Tim Killingback, Viktor Müller, Beáta Oborny, Greg Velicer, Dita Vizoso, and the referees for helpful discussions and critical review of the manuscript. Support from the Swiss Science Foundation is gratefully acknowledged.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Maynard Smith J, Szathmáry E. The Major Transitions in Evolution. Oxford: Freeman; 1995. [Google Scholar]
- 2.Wahl L M. J Theor Biol. 2002;219:371–388. doi: 10.1006/jtbi.2002.3133. [DOI] [PubMed] [Google Scholar]
- 3.Koufopanou V, Bell G. Proc R Soc London Ser B. 1993;254:107–113. [Google Scholar]
- 4.Hurst L D. J Theor Biol. 1990;144:429–443. doi: 10.1016/s0022-5193(05)80085-2. [DOI] [PubMed] [Google Scholar]
- 5.Bonner J T. Integr Biol. 1998;1:27–36. [Google Scholar]
- 6.Bonner J T. First Signals: The Evolution of Multicellular Development. Princeton: Princeton Univ. Press; 2000. [Google Scholar]
- 7.Kerszberg M, Wolpert L. J Theor Biol. 1998;193:535–537. doi: 10.1006/jtbi.1998.0714. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro J A. Annu Rev Microbiol. 1998;52:81–104. doi: 10.1146/annurev.micro.52.1.81. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser D. Annu Rev Genet. 2001;35:103–123. doi: 10.1146/annurev.genet.35.102401.090145. [DOI] [PubMed] [Google Scholar]
- 10.Crespi B J. Trends Ecol Evol. 2001;16:178–183. doi: 10.1016/s0169-5347(01)02115-2. [DOI] [PubMed] [Google Scholar]
- 11.Shimkets L J. Microbiol Rev. 1990;54:473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao L, Levin B R. Proc Natl Acad Sci USA. 1981;78:6324–6328. doi: 10.1073/pnas.78.10.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfeiffer T, Schuster S, Bonhoeffer S. Science. 2001;292:504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 14.Helling R B. J Bacteriol. 2002;184:1041–1045. doi: 10.1128/jb.184.4.1041-1045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stucki J W. Eur J Biochem. 1980;109:269–283. doi: 10.1111/j.1432-1033.1980.tb04792.x. [DOI] [PubMed] [Google Scholar]
- 16.Waddell T G, Repovic P, Meléndez-Hevia E, Heinrich R, Montero F. Biochem Educ. 1997;25:204–205. [Google Scholar]
- 17.Pfeiffer T, Bonhoeffer S. Z Phys Chem. 2002;216:51–63. [Google Scholar]
- 18.Hamilton W D. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 19.Taylor P D. Evol Ecol. 1992;6:352–356. [Google Scholar]
- 20.Wilson D S, Pollock G B, Dugatkin L A. Evol Ecol. 1992;6:331–341. [Google Scholar]
- 21.Frank S A. Foundations of Social Evolution. Princeton: Princeton Univ. Press; 1998. [Google Scholar]
- 22.West S A, Pen I, Griffin A S. Science. 2002;296:72–75. doi: 10.1126/science.1065507. [DOI] [PubMed] [Google Scholar]
- 23.Voet D, Voet J G. Biochemistry. New York: Wiley; 1995. [Google Scholar]
- 24.van Dijken J P, Weusthuis R A, Pronk J T. Antonie Leeuwenhoek. 1993;63:343–352. doi: 10.1007/BF00871229. [DOI] [PubMed] [Google Scholar]
- 25.Fiechter A, Gmunder F K. Adv Biochem Eng Biotechnol. 1989;39:1–28. doi: 10.1007/BFb0051950. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton W D, May R M. Nature. 1977;269:578–581. [Google Scholar]
- 27.Gandon S, Roussett F. Proc R Soc London Ser B. 1999;266:2507–2513. doi: 10.1098/rspb.1999.0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irwin A J, Taylor P D. Theor Popul Biol. 2000;58:321–328. doi: 10.1006/tpbi.2000.1490. [DOI] [PubMed] [Google Scholar]
- 29.Velicer G J, Kroos L, Lenski R E. Proc Natl Acad Sci USA. 1998;95:12376–12380. doi: 10.1073/pnas.95.21.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velicer G J, Kroos L, Lenski R E. Nature. 2000;404:598–601. doi: 10.1038/35007066. [DOI] [PubMed] [Google Scholar]
- 31.Shimkets L J. J Bacteriol. 1986;166:837–841. doi: 10.1128/jb.166.3.837-841.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schütte K H. New Phytol. 1956;55:164–182. [Google Scholar]
- 33.Persson C, Olsson S, Jansson H. FEMS Microbiol Ecol. 2000;31:47–51. doi: 10.1111/j.1574-6941.2000.tb00670.x. [DOI] [PubMed] [Google Scholar]
- 34.Plaskitt K A, Chater K F. Philos Trans R Soc London B. 1995;347:105–121. [Google Scholar]
- 35.Olsson S, Jennings D H. Exp Mycol. 1991;15:302–309. [Google Scholar]
- 36.Pepper J, Smuts B B. Am Nat. 2002;160:205–213. doi: 10.1086/341018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.