Abstract
Dystroglycan is a protein which binds directly to two proteins defective in muscular dystrophies (dystrophin and laminin α2) and whose own aberrant post-translational modification is the common aetiological route of neuromuscular diseases associated with mutations in genes encoding at least six other proteins (POMT1, POMT2, POMGnT1, LARGE, FKTN and FKRP). It is surprising, therefore, that to our knowledge no mutations of the human dystroglycan gene itself have yet been reported. In this study, we describe a patient with a heterozygous de novo deletion of a ∼2-Mb region of chromosome 3, which includes the dystroglycan gene (DAG1). The patient is a 16-year-old female with learning difficulties, white matter abnormalities, elevated serum creatine kinase, oral-motor dyspraxia and facial hypotonia but minimal clinically significant involvement of other muscles. As these symptoms are a subset of those observed in disorders of dystroglycan glycosylation (muscle–eye–brain disease and Warker–Warburg syndrome), we assess the likely contribution to her phenotype of her heterogosity for a null mutation of DAG1. We also show that the transcriptional compensation observed in the Dag1+/− mouse is not observed in the patient. Although we cannot show that haploinsufficiency of DAG1 is the sole cause of this patient's myopathy and white matter changes, this case serves to constrain our ideas of the severity of the phenotypic consequences of heterozygosity for null DAG1 mutations.
Keywords: dystroglycan, muscular dystrophy, learning difficulties, white matter, oral-motor dyspraxia
Introduction
Dystroglycan is an intriguing molecule with a fascinatingly complex biogenesis, a large number of interacting partners, and a wide-reaching biological involvement in a broad range of processes.1 Although transcribed and translated from a single gene, DAG1, dystroglycan is cleaved post-translationally to generate two polypeptides; a highly glycosylated extracellular subunit, α-dystroglycan, and a transmembrane subunit, β-dystroglycan.2 This cleavage is essential3 and may occur by autoproteolysis.4 Glycosylation appears to be of a highly specialised nature, such that the enzymatic pathway involved has been conserved for 500 million years of metazoan evolution, apparently for the sole purpose of modifying this single protein.5 This post-translational modification pathway involves at least six proteins,6 namely four glycosyltransferases – protein-O-mannosyltransferases 1 and 2 (POMT1, POMT2), protein-O-mannose β-1,2-N-acetylglucosaminyltransferase 1 (POMGnT1), acetylglucosaminyltransferase-like protein (LARGE) – and two probable phosphotransferases – fukutin and fukutin-related protein (FKTN, FKRP). Mutations in the genes encoding these proteins can give rise to disorders ranging from a late-onset limb-girdle muscular dystrophy (LGMD2I, LGMD2K and LGMD2M-2O)7 through congenital muscular dystrophies (MDC1C, MDC1D and FCMD) to muscle–eye–brain disease (MEB) and Walker–Warburg syndrome (WWS).7 Although the milder disorders result in an apparently simple myopathy reminiscent of the DMD–BMD spectrum,8 FCMD, MEB and WWS also have a variety of ocular and brain defects (including cobblestone lissencephaly, white matter abnormalities, learning difficulties, agenesis of the corpus callosum, myopia, cataracts and microphthalmia).9, 10 The relationship between degree of dystroglycan hypoglycosylation and phenotypic severity is significant but complex.11
Dystroglycan is known to bind to a number of intracellular and extracellular partners. Within the cell, the C-terminal tail of β-dystroglycan interacts with dystrophin, utrophin, DRP2 and other signalling molecules1 while, outside the cell, the glycosyl residues of α-dystroglycan bind to the laminin globular (LG) domains of molecules such as laminin α2 (merosin),2 neurexin,12 perlecan13 and agrin.14 Dystroglycan (through its sugar moieties) is also an important cell-surface receptor for several pathogens (Mycobacterium leprae,15 several Old World arenaviruses16). The majority of our ideas regarding the function of dystroglycan, based on the phenotypes of mouse17, 18, 19, 20, 21 and fruit fly dystroglycan mutants and human glycosylation defect patients, involve a role in the establishment and/or maintenance of basement membranes and their interaction with cell surfaces.
Homozygous null mutation of the dystroglycan gene (Dag1) in mouse is incompatible with life, resulting in resorption of mutant embryos by embryonic day 10.5, probably because of the developmental failure of the extraembryonic Reichert's membrane;21 similar problems underlie the lethality of the Fktn-null mouse.22 Conditional ablation of dystroglycan in differentiated skeletal muscle gives rise to a very mild myopathy (as dystroglycan is still expressed in satellite cells and at the neuromuscular and myotendinous junctions).17 Ablation of dystroglycan in the brain recapitulates the lissencephaly observed in the severe human dystroglycanopathy patients, and reveals that these may arise through failure of the glia limitans;18 other dystroglycanopathy-like structural abnormalities are also observed, and hippocampal long-term potentiation (a correlate of learning and memory) is impaired. Ablation in Schwann cells causes loss of DRP2 and dystrophin Dp116 complexes, abnormal myelination and node of Ranvier structure, and an evident peripheral neuropathy.19 Ablation of dystroglycan in all embryonic tissues combines all features of the above to give a severe WWS-like phenotype (lissencephaly, microphthalmia, tremor, small size, congenital muscular dystrophy, high frequency of neonatal death).20 Constitutive heterozygosity for the Dag1 null mutation seems to have no gross phenotypic effect on the mouse.21
We here describe a patient with a phenotype which is a subset of the symptoms of the WWS/MEB spectrum. She has a heterozygous de novo deletion which includes the DAG1 gene. We discuss the implications of this case for the spectrum of dystroglycanopathic phenotypes.
Materials and methods
Cytogenetics
DNA from the proband and her parents was tested for genome imbalance using oligonucleotide arrays with 44 000 probes across the genome (Agilent Technologies, Santa Clara, CA, USA). Hybridisation was carried out according to the manufacturer's recommendations, and the commercial analysis software was used, incorporating a 3-probe cut-off for imbalance flagging. The database of genomic variants (http://projects.tcag.ca/ variation/)23 was used to identify putatively benign copy number variants (CNVs).
Quantitative RT-PCR
Patient muscle RNA was prepared from 100 mg of vastus lateralis biopsy material by homogenising in 1 ml of QIAzol (RNA extraction kit, QIAGEN, Germantown, MD, USA) and subjecting to RNA extraction according to the manufacturers' instructions. Human muscle RNA purchased from Ambion (Austin, TX, USA) was used as a normal control sample. RNA was quantified using an RNA Nano Chip on an Agilent 2100 Bioanalyser (Agilent Technologies). The RNA samples were used in a quantitative nested RT-PCR using SYBR green detection in an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA; see Supplementary Information for primer sequences and detailed method). For each amplimer, measurements of threshold cycle (Ct) were used to infer initial template copy number when compared with Ct measurements of the respective serially diluted standard. Each datum plotted is the mean of three measurements (±SD) normalised to α-dystrobrevin and with the normal muscle expression level set to 100%.
Results
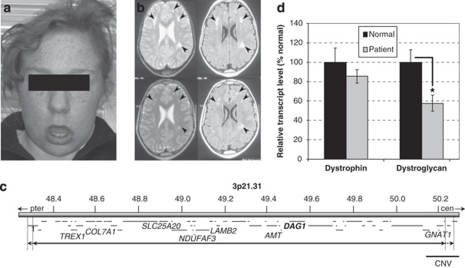
In this study, we describe a 16-year-old female patient with severe learning difficulties, facial hypotonia (Figure 1a), raised serum creatine kinase (CK) levels, oral-motor dyspraxia and subcortical white matter anomalies (Figure 1b – see Supplementary Information for a comprehensive description). A vastus lateralis muscle biopsy taken at the age of 7 years showed nonspecifically abnormal findings with some atrophic fibres and type 1 fibre predominance; occasional staining for neonatal myosin suggested a small amount of fibre regeneration (Supplementary Figure 1). Immunohistochemical staining for α-dystroglycan, laminins α2 and α5, dystrophin, utrophin and α-, β-, γ- and δ-sarcoglycans appeared normal. No evidence of inflammation or necrosis was reported, and mitochondrial staining was normal.
Figure 1.
(a) Facial features of the patient, aged 16 years, showing facial hypotonia with everted lower lip and protruding tongue. (b) Axial magnetic resonance images of the patient's brain at age 5 years (upper panels) and 11 years (lower panels). T2-weighted (left hand panels) and FLAIR (fluid-attenuated inversion recovery; right hand panels) images show mild ventricular dilatation and patchy, non-progressive high signal intensity changes in the subcortical white matter of both cerebral hemispheres (arrowheads), relatively symmetrical and slightly more marked in frontal regions. (c) Overview of the deleted region, del(3)(p21.31p21.31)(48286183–50219661)dn (solid line – minimum deletion; dashed lines – maximum deletion). The 65 genes (short horizontal lines) are listed in detail in Supplementary Table 4. The DAG1 gene (bold), seven genes implicated in human genetic disease, and a putatively benign CNV are labelled. (d) Levels of dystrophin and dystroglycan transcripts in normal and patient muscle. Columns show means of three measurements (±SD) normalised to α-dystrobrevin and with the normal muscle expression level set to 100%. *P<0.01.
Previous tests had revealed the patient to have a grossly normal 46, XX karyotype, and tests for subtelomeric deletion and the FRAXA, FRAXE and DMPK expansions proved negative. Array CGH analysis of the proband's DNA, however, identified a heterozygous ∼1.9-Mb deletion of material in the short arm of chromosome 3, band p21.31, as her only unusual copy number variation. The minimum extent of the deletion (as defined by the last deleted probes) is chr3:48286183–50219661 and the maximum extent chr3:48260361–50252191 (as defined by the first non-deleted probes; NCBI Build 36). The telomeric breakpoint therefore lies within the ZNF589 gene and the centromeric breakpoint in either SLC38A3 or GNAI2. A custom multiplex ligation-dependent probe amplification (MLPA) assay was used to independently confirm the deletion in the patient (probe site – 3:49126936); the MLPA assay also showed that the deletion was not present in either parent and has therefore occurred de novo in the proband. No clearly overlapping deletion was found on the DECIPHER database (https://decipher.sanger.ac.uk) or ECARUCA (http://agserver01.azn.nl:8080/ecaruca/ecaruca.jsp). The patient's details have been submitted to DECIPHER (patient number 251232).
Bioinformatic analysis of the maximally deleted region showed it to contain 62 protein-coding genes and three miRNA genes (see Supplementary Table 4 for a detailed assessment of each gene). Of these, 6 cause recessive disease in humans (with no reported heterozygous null phenotype), a further 6 have normal or almost normal phenotypes in homozygous null mice, and a further 10 have normal phenotypes in heterozygous null mice. Thus, for 22 of the genes there is reason not to expect haploinsufficient phenotype in humans. For most of the remainder, a broad biochemical function is either known or inferred, but the specific organ system(s) in which they act is unknown. In addition, eight of the genes are deleted in putatively benign CNVs found in the normal population and can therefore also be discounted. However, in the approximate centre of the deleted region lies DAG1, whose conditional knockout in mouse and aberrant post-translational modification in humans causes symptoms strikingly reminiscent of our patient.
To assess the effect of a heterozygous deletion of the entire DAG1 gene on DAG1 transcript levels (especially given the evidence from Dag1+/− mice for a degree of transcriptional compensation), we performed quantitative RT-PCR on RNA extracted from the muscle biopsy material. This shows that normalised levels of DAG1 mRNA in the patient's muscle are approximately 60% of those from wild-type muscle (Figure 1d; P<0.01, two-tailed t-test). We were not able to accurately assess protein levels; immunohistochemistry using antibodies against α-dystroglycan core protein, α-dystroglycan glycosyl moieties (IIH6) and β-dystroglycan showed normal staining (see Supplementary Figure 2), consistent with the limited quantitative properties of this technique. There was insufficient material to perform a western blot, which would also be unlikely to distinguish between 100 and 60% of wild-type protein levels. We cannot, therefore, ascertain the levels of dystroglycan protein in the patient. In an attempt to rule out a recessive effect on the DAG1 gene, we amplified and sequenced DAG1 cDNA corresponding to the patient's remaining, intact, allele (see Supplementary Information). We found this to be normal in sequence and structure.
Discussion
To our knowledge, this is the first description of a potentially pathogenic mutation of the DAG1 gene in humans. The patient presented here is essentially heterozygous for a null mutation of DAG1, and this gives us an opportunity to assess the consequences of this for humans. Although the mouse Dag1 null homozygote dies before implantation in the uterus, the corresponding heterozygote is described as phenotypically normal. It is noteworthy that the analysis of the heterozygote mouse shows that muscle dystroglycan protein levels are indistinguishable from normal (although western blots may have poor discrimination in the one- to twofold range) and that muscle Dag1 transcript levels are ∼80–90% of wild-type levels (by northern blot), suggesting some form of compensation.21 In our human heterozygote, we observe a much lesser degree of compensation (∼60% of normal), which may account for the observed phenotypic difference from the mouse. In addition, we note that compensation in the mouse may be tissue specific, that the relationship between transcript levels and protein levels is in turn likely to be tissue specific and complex, and that a neurological phenotype of the type observed in our patient may not be so evident in the cursory analysis of the Dag1+/− mouse.21
A key question to be addressed in this case is the extent to which haploinsufficiency for DAG1 is responsible for the patient's phenotype. The case for a major contribution by DAG1 rests largely on the similarity between the patient's phenotype and a subset of the symptoms evinced by MEB/WWS patients. The myopathy, which in the latter is pronounced, congenital and global, is understandably milder in our patient, who has limited myopathic signs in her leg muscle (as shown by the histopathology of her muscle biopsy), frank muscle weakness only in her face and possibly postural muscles, and a chronically elevated serum CK level. Of the brain malformations observed in MEB/WWS, our patient has subcortical white matter abnormalities and ventricular dilatation,9, 10 but not the more severe structural anomalies such as lissencephaly. She also has considerable learning difficulties, with significant speech delay compounded by oral-motor dyspraxia. Two other genes in the deleted region, namely TREX1 and LAMB2, are linked to relevant phenotypes. Null mutations in TREX1 give rise to recessive Aircardi–Goutière syndrome (OMIM 225750), which includes white matter anomalies; however, these are invariably calcified, a feature excluded in our patient. Null mutations in LAMB2 cause recessive Pierson syndrome (OMIM 609049), which includes severe muscle hypotonia associated with aberrant neuromuscular junctions; however, none of the ocular or renal symptoms of Pierson syndrome are observed in our patient (although hemizygosity for LAMB2 may exacerbate the consequences of hemizygosity for DAG1). Naturally, we cannot exclude the possibility that the deletion may lead to haploinsufficiency or expression of a pre-existing recessive allele in one or several of the other hemizygous genes.
It is difficult to extrapolate one's expectations of a phenotype from a situation in which a normal amount of core dystroglycan polypeptide is synthesised but is all aberrantly glycosylated (WWS/MEB) to one in which only 50–60% of the normal amount of dystroglycan is produced but is all correctly glycosylated (this case). The two instances presumably reflect the consequences of qualitative and quantitative change, respectively (though aberrant glycosylation can also result in a quantitative reduction in the final steady-state levels of dystroglycan). Like other haploinsufficiency disorders, the detailed cell-autonomous outcome is also likely to be dependent on cis and trans genetic modifiers, environmental factors and local stochastic phenomena. In summary, the haploinsufficient phenotype observed here represents an interesting subset of the WWS/MEB pattern, in that learning difficulties, white matter anomalies and facial hypotonia are observed, but severe global myopathy, lissencephaly and eye defects are not. This is reminiscent of the respectively severe and mild effects of dystroglycan ablation in the brain and skeletal muscle of the mouse,17, 18 and a recent paper suggests that similar combinations of severe dystroglycanopathy-like CNS features and mild skeletal myopathy are possible in humans.24
Although we cannot exclude effects from genes other than DAG1 in this patient, this case places an upper bound on the severity of the phenotypic consequences of heterozygosity for null DAG1 mutations in humans. We would suggest that the possibility of heterozygosity for null DAG1 mutations should be analysed in other patients with this phenotype.
Acknowledgments
We are grateful to our clinical colleagues Dr Charu Deshpande, Dr Elizabeth Wraige, Dr Alan Beattie and Dr Shehla Mohammed for their advice, and to Mr Stefan Buk (Department of Clinical Neuropathology, King's College Hospital) and Professor Caroline Sewry (RJAH Orthopaedic Hospital) for immunohistochemistry and advice on the paper. We thank the patient and her family for their cooperation. SVB and RGR are supported by the Muscular Dystrophy Campaign and the London Law Trust.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Jayasinha V, Nguyen HH, Xia B, Kammesheidt A, Hoyte K, Martin PT. Inhibition of dystroglycan cleavage causes muscular dystrophy in transgenic mice. Neuromuscul Disord. 2003;13:365–375. doi: 10.1016/s0960-8966(03)00040-3. [DOI] [PubMed] [Google Scholar]
- Akhavan A, Crivelli SN, Singh M, Lingappa VR, Muschler JL. SEA domain proteolysis determines the functional composition of dystroglycan. FASEB J. 2008;22:612–621. doi: 10.1096/fj.07-8354com. [DOI] [PubMed] [Google Scholar]
- Haines N, Seabrooke S, Stewart BA. Dystroglycan and protein O-mannosyltransferases 1 and 2 are required to maintain integrity of Drosophila larval muscles. Mol Biol Cell. 2007;18:4721–4730. doi: 10.1091/mbc.E07-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntoni F, Torelli S, Brockington M. Muscular dystrophies due to glycosylation defects. Neurotherapeutics. 2008;5:627–632. doi: 10.1016/j.nurt.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JC. The 2009 version of the gene table of neuromuscular disorders. Neuromuscul Disord. 2009;19:77–98. doi: 10.1016/j.nmd.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Guglieri M, Straub V, Bushby K, Lochmuller H. Limb-girdle muscular dystrophies. Curr Opin Neurol. 2008;21:576–584. doi: 10.1097/WCO.0b013e32830efdc2. [DOI] [PubMed] [Google Scholar]
- Clement E, Mercuri E, Godfrey C, et al. Brain involvement in muscular dystrophies with defective dystroglycan glycosylation. Ann Neurol. 2008;64:573–582. doi: 10.1002/ana.21482. [DOI] [PubMed] [Google Scholar]
- Cormand B, Pihko H, Bayes M, et al. Clinical and genetic distinction between Walker-Warburg syndrome and muscle-eye-brain disease. Neurology. 2001;56:1059–1069. doi: 10.1212/wnl.56.8.1059. [DOI] [PubMed] [Google Scholar]
- Jimenez-Mallebrera C, Torelli S, Feng L, et al. A comparative study of alpha-dystroglycan glycosylation in dystroglycanopathies suggests that the hypoglycosylation of alpha-dystroglycan does not consistently correlate with clinical severity. Brain Pathol. 2009;19:596–611. doi: 10.1111/j.1750-3639.2008.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HB, Ali AA, Daggett DF, Rauvala H, Hassell JR, Smalheiser NR. The relationship between perlecan and dystroglycan and its implication in the formation of the neuromuscular junction. Cell Adhes Commun. 1998;5:475–489. doi: 10.3109/15419069809005605. [DOI] [PubMed] [Google Scholar]
- Sugiyama J, Bowen DC, Hall ZW. Dystroglycan binds nerve and muscle agrin. Neuron. 1994;13:103–115. doi: 10.1016/0896-6273(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Rambukkana A, Yamada H, Zanazzi G, et al. Role of alpha-dystroglycan as a Schwann cell receptor for Mycobacterium leprae. Science. 1998;282:2076–2079. doi: 10.1126/science.282.5396.2076. [DOI] [PubMed] [Google Scholar]
- Cao W, Henry MD, Borrow P, et al. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- Cohn RD, Henry MD, Michele DE, et al. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell. 2002;110:639–648. doi: 10.1016/s0092-8674(02)00907-8. [DOI] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, et al. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Satz JS, Barresi R, Durbeej M, et al. Brain and eye malformations resembling Walker-Warburg syndrome are recapitulated in mice by dystroglycan deletion in the epiblast. J Neurosci. 2008;28:10567–10575. doi: 10.1523/JNEUROSCI.2457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RA, Henry MD, Daniels KJ, et al. Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag1-null mice. Hum Mol Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- Kurahashi H, Taniguchi M, Meno C, et al. Basement membrane fragility underlies embryonic lethality in fukutin-null mice. Neurobiol Dis. 2005;19:208–217. doi: 10.1016/j.nbd.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Iafrate AJ, Feuk L, Rivera MN, et al. Detection of large-scale variation in the human genome. Nat Genet. 2004;36:949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- Okanishi T, Ishikawa T, Kobayashi S, et al. Bilateral occipital cortical dysplasia and white matter T2 hyperintensity with mild non-specific myopathy: two sibling cases Brain Dev 2010. doi: 10.1016/j.braindev.2009.11.006PMID: 20022722. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.