Abstract
Interleukin (IL)-2/IL-2R signalling promotes proliferation and survival of activated T cells and has an essential non-redundant role in the production of regulatory T cells. Associations with different autoimmune diseases of polymorphisms in a linkage disequilibrium block in which the IL2/IL21 genes map (4q27), and also in genes encoding the IL2RA and IL2RB subunits (located in 10p15 and 22q13, respectively), were identified through genome-wide studies. Polymorphisms in these three genes were studied in 430 multiple sclerosis (MS) patients and in 550 ethnically matched controls from Madrid (Spain). Replication and meta-analysis with results from an independent cohort of 771 MS patients and 759 controls from Andalucía (Spain) confirmed the association of polymorphisms in the IL2RA gene (PMantel–Haenszel, odds ratio (OR)M–H (95% confidence interval, CI) for rs2104286: 0.0001, 0.75 (0.65–0.87); for rs11594656/rs35285258: 0.004, 1.19 (1.06–1.34); for rs41295061: 0.03, 0.77 (0.60–0.98)); showed a trend for association of the IL2/IL21 rs6822844 (PM–H=0.07, ORM–H (95% CI)=0.86 (0.73–1.01)), but did not corroborate the association for IL2RB. Regression analyses of the combined Spanish cohort revealed the independence of two IL2RA association signals: rs2104286 and rs11594656/rs35285258. The relevant role of the IL2RA gene on MS susceptibility adds support to its common effect on autoimmune risk and the suggestive association of IL2/IL21 warrants further investigation.
Keywords: multiple sclerosis, IL2, IL2RA, IL2RB, polymorphism, susceptibility
INTRODUCTION
Genome-wide association studies have recently identified risk variants in 4q27 that increased susceptibility towards several autoimmune diseases such as coeliac disease,1 type I diabetes2 or psoriasis.3 Those polymorphisms are located in a 480 kb linkage disequilibrium block that harbours interleukin 2 (IL2) and IL21 genes, among others. Therefore, this locus is emerging as a shared susceptibility factor for diseases with an immune aetiology. Owing to the correction threshold imposed on these pangenomic studies, some associations do not reach significance on a first-hit level, but they are evidenced in follow-up studies, as has been demonstrated for the role of the IL2–IL21 locus on rheumatoid arthritis (RA).4
Interleukin 2 was initially identified as an autocrine secretory product from activated T cells with growth factor properties. Later, it was found that IL-2 elicits T-cell proliferation, survival and differentiation of effectors (Th1/Th2). However, the main non-redundant function of IL-2 consists of maintaining peripheral T-cell tolerance, and the impairment of regulatory T cells is thought to be the underlying cause of autoimmunity in the absence of IL-2. This cytokine is also able to sensitize activated T cells to undergo apoptosis or activation-induced cell death, limiting the immune response.5 The binding of IL-2 to IL-2Rα drives the association of this subunit with the IL-2Rβ- and γ-chain to form the high-affinity heterotrimeric IL-2 receptor.6 Polymorphisms within the genes encoding two different subunits of the receptor, IL2RA and IL2RB, have also been associated with greater predisposition to type I diabetes,7 RA8 or multiple sclerosis (MS).9, 10
Multiple sclerosis is a complex disease presumed to be autoimmune in nature and with a clear inflammatory and demyelinating outcome. A genome-wide association scan pointed to the role of the IL2RA gene in MS predisposition,9 and that association was recently confirmed in a large multicentre study.11 The IL2RA gene had previously shown its relevance in the development of MS.10 Moreover, a polymorphism in the IL2 promoter was found to influence MS risk in a classical candidate-gene approach,12 although in genome-wide studies, the associations of either IL2 or IL2RB polymorphisms with MS did not exceed the significance threshold.9, 13
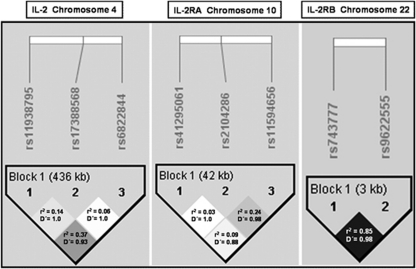
The association of IL2, IL2RA and IL2RB genes with multiple autoimmune diseases might suggest a common mechanism in their pathogenesis and we aimed at studying polymorphisms previously associated with different autoimmune conditions. Dissecting the specific variant responsible for the IL2 effect has been hampered by the tight linkage disequilibrium of the 4q27 locus, and therefore we genotyped three SNPs: rs6822844 and rs11938795, associated with coeliac disease,1 and one polymorphism associated with diabetes, rs17388568.2 Two independent signals of the IL2RA gene were identified by fine mapping of the region, and their minor alleles were related to inherited lower circulating levels of the soluble IL-2RA protein.7 We selected tagging SNPs for these signals, rs41295061 and rs11594656, and one polymorphism (rs2104286) previously associated with MS.9 Polymorphisms within the genes encoding IL-2R subunits α (rs2104286) and β (rs743777) were both associated with RA and type I diabetes (T1D) in the genome-wide study performed by the Wellcome Trust Consortium.8 rs9622555, located in the same linkage disequilibrium block as rs743777 (see Figure 1), was selected to eventually support the data of the latter.
Figure 1.
Linkage disequilibrium map of the polymorphisms studied within the IL2, IL2RA and IL2RB genes.
METHODS
A total of 430 Spanish MS patients and 550 ethnically matched controls, mostly blood donors and staff, were consecutively recruited from the Hospital Clínico (Madrid), and an independent Spanish cohort from Andalucía was also studied including individuals from Hospital Virgen de las Nieves, Hospital Clínico San Cecilio, Banco de Sangre (Granada), Hospital Carlos Haya (Málaga) and Hospital Virgen Macarena (Sevilla). The Ethics Committees of these Hospitals approved the study. Patients were diagnosed on the basis of the Poser criteria14 and were included in the study after informed consent. Clinical features of most of them have been published previously.15, 16 In the MS cohort from Madrid (35% men and 65% women), with a mean age at onset of 29 years and the age of patients ranging from 16 to 79 years, 208 individuals were carriers of the major susceptibility factor for MS (HLA-DRB1*1501). The mean Expanded Disability Status Scale (EDSS) was 3.5 and ∼10% of patients had a father, mother or a sibling with MS. The clinical forms were distributed as follows: 32 primary progressive, 46 secondary progressive and 321 relapsing-remitting (for the remaining 31 patients, we had no data). The Andalusian cohort recruited 759 healthy controls (32% men and 68% women) and 771 MS patients (31% men and 69% women) with a mean age at onset of 29 years and age ranging from 15 to 71 years. The clinical forms of the MS cohort were 255 secondary progressive and 516 relapsing-remitting.
Genotyping of samples was carried out with TaqMan Assays from Applied Biosystems in a 7900HT Fast Real-Time PCR system, under the conditions recommended by the manufacturer (Applied Biosystems, Foster City, CA, USA). Genotyping call-rate success was over 96% in MS cases and controls for every tested polymorphism.
The statistical analysis to compare allelic and genotypic distributions was performed using χ2-test or Fisher's exact test (when expected values were below 5) included in a standard statistical package (Epi Info v. 6.02; World Health Organization, Geneva, Switzerland). Odds ratios (ORs) were calculated, and their 95% confidence intervals (CIs) were estimated using the Cornfield method. Haplotypic frequencies were estimated with the expectation–maximization algorithm implemented in the Haploview v.4.1 software. A combined analysis of the two cohorts was performed using the Mantel–Haenszel test (also implemented on Epi Info software). Linkage disequilibrium was measured by calculating two parameters, r2 and D′ (Figure 1).
RESULTS
Polymorphisms in the IL2, IL2RA and IL2RB genes were genotyped in the samples from Madrid (Table 1). None of the tested polymorphisms significantly deviated from Hardy–Weinberg predictions. The frequency of carriers of the minor allele for rs6822844 was significantly lower in MS patients compared with controls (P=0.03; OR (95% CI)=0.72 (0.52–0.98)). No significant differences were found in the other two IL2 polymorphisms, which only showed low to modest genetic equivalence with rs6822844 (r2=0.06 and 0.37, Figure 1). The minor allele frequency for rs2104286, located in intron 1 of the IL2RA gene, was significantly lower in MS patients compared with controls. Two other polymorphisms located upstream of the IL2RA gene were studied, and a similar protective trend was found for rs41295061. The frequency of the mutant genotype for rs11594656 was higher in MS patients (AA vs (TA+TT): P=0.02; OR (95% CI)=1.61 (1.05–2.48)). In addition, two markers, located within 10 kb upstream of the IL2RB transcription origin, were analysed. The frequency of mutant genotypes for both SNPs in this gene (r2=0.85) was lower in the patient group than in controls (rs743777, GG vs (GA+AA): P=0.07; OR (95% CI)=0.69 (0.45–1.06) and rs9622555, TT vs (TG+GG): P=0.11; OR (95% CI)=0.70 (0.40–1.11)). Minor allele frequencies of these IL2RB polymorphisms were also lower in patients than in controls (Table 1).
Table 1. Genotype and allele frequencies of IL2, IL2RA and IL2RB polymorphisms in MS patients and healthy controls recruited from Madrid and Andalucía, and overall Mantel–Haenszel analyses of the pooled Spanish cohorts.
| IL2RA | ||||
|---|---|---|---|---|
| MS Madrid | Controls Madrid | MS Andalucía16 | Controls Andalucía16 | |
| rs11938795 | ||||
| TT | 231 (56%) | 293 (54%) | — | — |
| TC | 155 (38%) | 206 (38%) | — | — |
| CC | 26 (6%) | 47 (9%) | — | — |
| T | 617 (75%) | 792 (73%) | — | — |
| C | 207 (25%) | 300 (27%) | — | — |
| Allelic P | 0.25 | |||
| Allelic OR (95% CI) | 0.89 (0.72–1.09) | |||
| rs17388568 | ||||
| GG | 229 (54%) | 289 (54%) | — | — |
| GA | 162 (38%) | 214 (40%) | — | — |
| AA | 35 (8%) | 37 (7%) | — | — |
| G | 620 (73%) | 792 (73%) | — | — |
| A | 232 (27%) | 288 (27%) | — | — |
| Allelic P | 0.78 | |||
| Allelic OR (95% CI) | 1.03 (0.84–1.27) | |||
| rs6822844a | ||||
| GG | 334 (80%) | 405 (74%) | 598 (78%) | 717 (77%) |
| GT | 79 (19%) | 136 (25%) | 160 (21%) | 191 (21%) |
| TT | 6 (1%) | 8 (1%) | 10 (1%) | 21 (2%) |
| G | 747 (89%) | 946 (86%) | 1356 (88%) | 1625 (87%) |
| T | 91 (11%) | 152 (14%) | 180 (12%) | 233 (13%) |
| Allelic P | 0.05 | 0.47 | ||
| Allelic OR (95% CI) | 0.76 (0.57–1.01) | 0.93 (0.75–1.15) | ||
| Allelic PMantel–Haenszel=0.076 | ||||
| Allelic ORMantel–Haenszel (95% CI)=0.86 (0.72–1.01) | ||||
| IL2RA | ||||
| MS Madrid | Controls Madrid | MS Andalucía16 | Controls Andalucía16 | |
| rs11594656b (rs35285258 in Andalucía) | ||||
| TT | 186 (45%) | 251 (46%) | 300 (39%) | 334 (45%) |
| TA | 172 (42%) | 244 (45%) | 366(47%) | 320 (43%) |
| AA | 56 (14%) | 48 (9%) | 105 (14%) | 82 (11%) |
| T | 544 (66%) | 746 (69%) | 966 (63%) | 988 (67%) |
| A | 284 (34%) | 340 (31%) | 576 (37%) | 484 (33%) |
| Allelic P | 0.17 | 0.01 | ||
| Allelic OR (95% CI) | 1.15 (0.94–1.40) | 1.22 (1.04–1.42) | ||
| Allelic PMantel–Haenszel=0.004 | ||||
| Allelic ORMantel–Haenszel (95% CI)=1.19 (1.05–1.33) | ||||
| rs41295061 | ||||
| CC | 381 (91%) | 479 (88%) | 678 (89%) | 650 (87%) |
| CA | 35 (8%) | 66 (12%) | 82 (11%) | 93 (12%) |
| AA | 1 (0%) | 2 (0%) | 1 (0%) | 3 (0%) |
| C | 797 (96%) | 1024 (94%) | 1438 (94%) | 1393 (93%) |
| A | 37 (4%) | 70 (6%) | 84 (6%) | 99 (7%) |
| Allelic P | 0.06 | 0.19 | ||
| Allelic OR (95% CI) | 0.68 (0.44–1.04) | 0.82 (0.60–1.12) | ||
| Allelic PMantel–Haenszel=0.03 | ||||
| Allelic ORMantel–Haenszel (95% CI)=0.77 (0.6–0.97) | ||||
| rs2104286 | ||||
| AA | 303 (72%) | 337 (62%) | 508 (67%) | 483 (64%) |
| AG | 108 (26%) | 183 (34%) | 224 (30%) | 238 (31%) |
| GG | 8 (2%) | 25 (5%) | 21 (3%) | 38 (5%) |
| A | 714 (85%) | 857 (79%) | 1240 (82%) | 1204 (79%) |
| G | 124 (15%) | 233 (21%) | 266 (18%) | 314 (21%) |
| Allelic P | 0.0002 | 0.03 | ||
| Allelic OR (95% CI) | 0.64 (0.50–0.82) | 0.82 (0.68–0.99) | ||
| Allelic PMantel–Haenszel=0.0001 | ||||
| Allelic ORMantel–Haenszel (95% CI)=0.75 (0.65–0.86) | ||||
| IL2RB | ||||
| MS Madrid | Controls Madrid | MS Andalucía | Controls Andalucía | |
| rs743777 | ||||
| AA | 190 (46%) | 242 (45%) | — | — |
| GA | 183 (44%) | 221 (41%) | — | — |
| GG | 40 (10%) | 72 (13%) | — | — |
| A | 563 (68%) | 705 (66%) | — | — |
| G | 263 (32%) | 365 (34%) | — | — |
| Allelic P | 0.30 | |||
| Allelic OR (95% CI) | 0.90 (0.74–1.10) | |||
| rs9622555 | ||||
| GG | 225 (54%) | 267 (49%) | 342 (46%) | 344 (51%) |
| GT | 160 (38%) | 217 (40%) | 335 (45%) | 266 (40%) |
| TT | 34 (8%) | 61 (11%) | 69 (9%) | 62 (9%) |
| G | 610 (73%) | 751 (69%) | 1019 (68%) | 954 (71%) |
| T | 228 (27%) | 339 (31%) | 473 (32%) | 390 (29%) |
| Allelic P | 0.06 | 0.12 | ||
| Allelic OR (95% CI) | 0.83 (0.68–1.02) | 1.14 (0.96–1.34) | ||
ORs referred to the minor allele.
Carrier T (Madrid cohort): P=0.03; OR (95% CI)=0.72 (0.52–0.98).
Carrier T (Madrid cohort): P=0.02; OR (95% CI)=0.62 (0.4–0.95).
To validate whether these markers were bona fide risk factors, we decided to analyse the polymorphisms showing any evidence of association with MS in the Madrid population, in an independent Spanish cohort from Andalucía. Once the homogeneity of the data was confirmed (homogeneity P>0.1), we performed a Mantel–Haenszel analysis of the significant results from the samples from Madrid pooled with those previously published for the Andalusian cohort16, 17 (Table 1). The IL2 polymorphism, rs6822844, showed a trend for association (P=0.076) and the three polymorphisms tested in the IL2RA gene yielded significant associations in the overall cohort. The IL2RB polymorphism rs9622555 was genotyped in the Andalusian cohort showing no association with MS. The genotypic distribution of rs9622555 showed heterogeneity between the cohorts from Madrid and Andalucía, and therefore the data obtained could not be merged.
Regression analyses of IL2RA polymorphisms revealed the independence of rs2104286 and rs11594656/rs35285258, but rs41295061 did not show an independent effect after adjustment for rs2104286 (see Table 2).
Table 2. Conditional logistic regression analyses for IL2RA polymorphisms with single-point allelic P<0.05 in the combined cohorts from Madrid and Andalucía (1247 MS cases and 1491controls).
| (a) Add locus to rs2104286 | (b) Add rs2104286 to locus | ||||||
|---|---|---|---|---|---|---|---|
| Locus | OR | (95% CI) | P-value | OR | (95% CI) | P-value | |
| rs41295061 | C | 1.00 | (0.76–1.31) | 0.98 | 0.74 | (0.63–0.87) | 0.0003 |
| rs35285258 | T | 1.14 | (1.01–1.29) | 0.03 | 0.80 | (0.69–0.93) | 0.0038 |
Regression analyses (a) adding rs41295061 and rs35285258 to rs2104286 and (b) adding rs2104286 to rs41295061 and rs35285258. Results for a model assuming multiplicative effects.
No significant differences were observed after stratification for the main susceptibility determinant in MS, the HLA-DRB1*1501 allele,18 or for age/gender in any of the studied polymorphisms (data not shown). The study of inferred haplotypes did not add any further information (data not shown).
Discussion
Genetic linkage analysis in the mouse model of MS identified an IL2-containing locus which affected disease severity.19 A decade later, treatment with an IL2-caspase3 chimeric protein of experimental autoimmune encephalitis EAE-induced mice caused a significant delay in disease onset by controlling lymphocyte reactivity through targeted apoptosis.20 Moreover, the cytokine profile of myelin crossreactive vs monospecific Epstein–Barr virus nuclear antigen 1-specific T cells differed in their capability to produce IL-2.21 Among the 27 tagging SNPs that very efficiently captured the common genetic variation in the IL2/IL21 linkage disequilibrium block, the minor allele of rs6822844 showed the strongest association with coeliac disease in three independent populations,1 in agreement with results in type I diabetes8 and RA,4 with a similar protective effect among these diseases. Our present data from the Madrid cohorts showed a borderline association of rs6822844 with MS (Table 1), and although the results from the other Spanish cohort from Andalucía17 did not reach significance, the combined data seem to follow the same trend of association of this IL2 polymorphism. Therefore, our data agree with the aforementioned results suggesting the importance of IL-2 in MS development, and corroboration of the association with this IL2 polymorphism should be pursued.
IL-2RA-specific humanized monoclonal antibody showed promising therapeutic effects in MS,22 probably through an immunoregulatory pathway involving the expansion of natural killer cells that are able to lyse activated autologous T cells.23 Strongly associated IL2RA markers were identified in a MS genome-wide study9 and then validated in independent populations,11, 24 in agreement with the present data. Maier et al25 showed an independent association of rs2104286 and rs35285258/rs11594656, and pointed out that the minor allele of rs2104286 confers susceptibility to both MS and type I diabetes, whereas the allele for rs35285258/rs11594656 confers protection towards diabetes,7 but susceptibility to MS. Maybe because of low statistical power, Alcina et al16 did not detect the independence of these signals; however, analysis of the pooled Spanish samples from Madrid and Andalucía now corroborate the independent effect of those polymorphisms originally described by Maier et al25 (Table 2). Recently, it has been demonstrated that differences in surface expression of the IL-2RA or CD25 protein are restricted to particular immune cell types and correlated with several haplotypes in the IL2RA region, underscoring the gene–phenotype association between protein levels on specific subsets of primary human immune cell types and autoimmune disease polymorphisms.26
As previously reported, in vivo administration of anti-IL-2RB mAb resulted in persistent MS symptoms in the mouse model,27 arguing in favour of a role of the IL2RB gene in the disease. Nevertheless, we could not replicate the association observed in Madrid in the other Spanish samples. The reason for this discrepancy may be an interspecies difference or else it could be due to an effect on a yet-undetermined subgroup of patients, which would explain the heterogeneous effect obtained in both Spanish cohorts.
The IL2–IL2R genes are attractive candidates as MS susceptibility factors. Regulatory T cells actively suppress autoreactive T cells in the periphery,28 and reduced regulatory T function has been detected in MS patients.29, 30 Our data replicate the reported independent effects of two polymorphisms in the IL2RA locus, but the studied IL2RB polymorphisms show no signs of an overall effect on MS risk. The association found in Graves' disease,31 as well as the one previously reported with T1D7, 32 and MS,9, 16 suggests that the IL2RA/CD25 region acts as a general susceptibility locus for autoimmune diseases. In fact, anti-CD25 daclizumab seems to be a promising therapy for the inhibition of inflammation and stabilization of MS progression.33 The IL2 region was recently associated with several autoimmune diseases. A suggestive signal of association is now found for rs6822844, which deserves replication to corroborate the borderline effect observed. Therefore, a similar scenario involving IL2 in MS risk seems to emerge. These facts are consistent with a major role for the IL-2/IL-2 receptor pathway in the control of autoimmunity.
Acknowledgments
We thank Carmen Martínez and M Angel García for their skilful technical assistance. Concepción Núñez holds a research contract from Fondo Investigaciones Sanitarias (CA06/0163) and Elena Urcelay works for the Fundación para la Investigación Biomédica-Hospital Clínico San Carlos. This study was supported by grants from: Alfonso Martín Escudero Foundation and Fondo Investigaciones Sanitarias FIS PI07/0353, FIS PI07/0369, PI08/1636 and Plan Nacional PN-SAF2006-02023.
The authors declare no conflict of interest.
References
- van Heel DA, Franke L, Hunt KA, et al. A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat Genet. 2007;39:827–829. doi: 10.1038/ng2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JA, Walker NM, Cooper JD, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Helms C, Liao W, et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008;4:e1000041. doi: 10.1371/journal.pgen.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhernakova A, Alizadeh BZ, Bevova M, et al. Novel association in chromosome 4q27 region with rheumatoid arthritis and confirmation of type 1 diabetes point to a general risk locus for autoimmune diseases. Am J Hum Genet. 2007;81:1284–1288. doi: 10.1086/522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vang KB, Farrar MA. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol Lett. 2007;114:1–8. doi: 10.1016/j.imlet.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CE, Cooper JD, Brusko T, et al. Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet. 2007;39:1074–1082. doi: 10.1038/ng2102. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium Genome-wide association study of 14 000 cases of seven common diseases and 3000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler DA, Compston A, Sawcer S, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- Matesanz F, Caro-Maldonado A, Fedetz M, et al. IL2RA/CD25 polymorphisms contribute to multiple sclerosis susceptibility. J Neurol. 2007;254:682–684. doi: 10.1007/s00415-006-0416-4. [DOI] [PubMed] [Google Scholar]
- International Multiple Sclerosis Genetics Consortium (IMSGC) Refining genetic associations in multiple sclerosis. Lancet Neurol. 2008;7:567–569. doi: 10.1016/S1474-4422(08)70122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matesanz F, Fedetz M, Collado-Romero M, et al. Allelic expression and interleukin-2 polymorphisms in multiple sclerosis. J Neuroimmunol. 2001;119:101–105. doi: 10.1016/s0165-5728(01)00354-x. [DOI] [PubMed] [Google Scholar]
- Baranzini SE, Galwey NW, Wang J, et al. Pathway and network-based analysis of genome-wide association studies in multiple sclerosis. Hum Mol Genet. 2009;18:2078–2090. doi: 10.1093/hmg/ddp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Martinez A, Santiago JL, Cenit MC, et al. IFIH1-GCA-KCNH7 locus: influence on multiple sclerosis risk. Eur J Hum Genet. 2008;16:861–864. doi: 10.1038/ejhg.2008.16. [DOI] [PubMed] [Google Scholar]
- Alcina A, Fedetz M, Ndagire D, et al. IL2RA/CD25 gene polymorphisms: uneven association with multiple sclerosis (MS) and type 1 diabetes (T1D) PLoS ONE. 2009;4:e4137. doi: 10.1371/journal.pone.0004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedetz M, Ndagire D, Fernandez O, et al. Multiple sclerosis association study with the TENR-IL2-IL21 region in a Spanish population. Tissue Antigens. 2009;74:244–247. doi: 10.1111/j.1399-0039.2009.01298.x. [DOI] [PubMed] [Google Scholar]
- Jersild C, Ammitzboll T, Clausen J, Fog T. Association between HL-A antigens and measles antibody in multiple sclerosis. Lancet. 1973;1:151–152. doi: 10.1016/s0140-6736(73)90224-9. [DOI] [PubMed] [Google Scholar]
- Encinas JA, Kuchroo VK. Genetics of experimental autoimmune encephalomyelitis. Curr Dir Autoimmun. 1999;1:247–272. doi: 10.1159/000060485. [DOI] [PubMed] [Google Scholar]
- Irony-Tur-Sinai M, Lichtenstein M, Brenner T, Lorberboum-Galski H. IL2-caspase3 chimeric protein controls lymphocyte reactivity by targeted apoptosis, leading to amelioration of experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2009;9:1236–1243. doi: 10.1016/j.intimp.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Lunemann JD, Jelcic I, Roberts S, et al. EBNA1-specific T cells from patients with multiple sclerosis cross react with myelin antigens and co-produce IFN-gamma and IL-2. J Exp Med. 2008;205:1763–1773. doi: 10.1084/jem.20072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JW, Burns JB, Bjorklund J, Klein J, Watt HE, Carlson NG. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology. 2007;69:785–789. doi: 10.1212/01.wnl.0000267662.41734.1f. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F, Fontaine B, Cournu-Rebeix I, et al. IL2RA and IL7RA genes confer susceptibility for multiple sclerosis in two independent European populations. Genes Immun. 2008;9:259–263. doi: 10.1038/gene.2008.14. [DOI] [PubMed] [Google Scholar]
- Maier LM, Lowe CE, Cooper J, et al. IL2RA genetic heterogeneity in multiple sclerosis and type 1 diabetes susceptibility and soluble interleukin-2 receptor production. PLoS Genet. 2009;5:e1000322. doi: 10.1371/journal.pgen.1000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendrou CA, Plagnol V, Fung E, et al. Cell-specific protein phenotypes for the autoimmune locus IL2RA using a genotype-selectable human bioresource. Nat Genet. 2009;41:1011–1015. doi: 10.1038/ng.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Ishida Y, Rifa'i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825–832. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period. J Exp Med. 2005;201:769–777. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp Immunol. 2007;147:412–418. doi: 10.1111/j.1365-2249.2006.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand OJ, Lowe CE, Heward JM, et al. Association of the interleukin-2 receptor alpha (IL-2Ralpha)/CD25 gene region with Graves' disease using a multilocus test and tag SNPs. Clin Endocrinol (Oxf) 2007;66:508–512. doi: 10.1111/j.1365-2265.2007.02762.x. [DOI] [PubMed] [Google Scholar]
- Vella A, Cooper JD, Lowe CE, et al. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am J Hum Genet. 2005;76:773–779. doi: 10.1086/429843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Howard T, Packer AN, et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch Neurol. 2009;66:483–489. doi: 10.1001/archneurol.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]