Abstract
The expansion of newborn screening (NBS) has been accompanied by debate about what benefits should be achieved and the role of parental discretion in their pursuit. The opportunity to inform parents of reproductive risks is among the most valued additional benefits gained through NBS, and assumes prominence where the primary goal of identifying a treatable condition is not assured. We reviewed 53 unique guidelines addressing prenatal, preconception and newborn screening to examine: (1) how generating reproductive risk information is construed as a benefit of screening; and (2) what conditions support the realization of this benefit. Most preconception and prenatal guidelines – where generating reproductive risk information is described as a primary benefit – required that individuals be given a ‘cascade of choices', ensuring that each step in the decision-making process was well informed, from deciding to pursue information about reproductive risks to deciding how to manage them. With the exception of three guidelines, NBS policy infrequently attended to the potential for reproductive benefits; further, most guidelines that acknowledged such benefits construed voluntarism narrowly, without attention to the choices attendant on receiving reproductive risk information. This review suggests that prenatal and preconception guidance identifies a coherent framework to support the pursuit of reproductive benefits through population screening programmes. Interestingly, attention to reproductive benefits is increasing among NBS guidance, yet reflection on how such benefits ought to be pursued remains limited. Traditional norms for NBS may require reconsideration where the remit of screening exceeds the primary goal of clinical benefits for infants.
Keywords: newborn screening, reproductive risk information, policy, ethics, informed decision making
Introduction
Newborn screening (NBS) is a premier example of the application of genomic discoveries to population health benefits. Traditionally, NBS programmes identified serious conditions where early detection and urgent presymptomatic treatment were necessary to avert serious clinical harm. The classic example is phenylketonuria, where the immediate detection of affected babies resulted in clinical benefit through lifelong dietary management, effectively preventing neurological devastation. In this context, NBS operated under the rubric of a ‘public health emergency' model,1 in which screening was mandatory or consent was otherwise implied.
Although these clinical goals remain, increased technological capacity means that expanded NBS programmes can now identify a broader range of conditions, including those for which treatment is not established, as well as benign carrier states or variants of uncertain clinical significance.2 In consequence, expansion has been accompanied by debates about the nature of benefit to be achieved. Advocates of expanded infant screening programmes argue for a wider interpretation of the notion of benefit.3 They maintain that screening for conditions in which clinical outcomes are unproven or limited provides information, and permits access to programmes that offer education and support.4, 5 The opportunity to inform parents and infants of future reproductive risks is among the most valued additional benefits to be achieved.4, 5, 6, 7, 8
Historically, ‘reproductive benefit' – that is, the potential benefit of learning reproductive risk information to support family planning – arose as a secondary outcome of the primary goal of identifying a treatable condition, and thus little attention was given to how such a benefit should be realized. Yet, there are several ways in which expanded NBS upsets this hierarchy of benefits.9 The first is in the case of expanded NBS panels that include conditions for which clear evidence of medical benefit is not established,2 such that the identification of reproductive risks assumes greater prominence.3, 4 A second way pertains to certain rare conditions, such as Duchenne muscular dystrophy, for which there is no medical treatment but for which early diagnosis permits the identification of reproductive risks.10, 11, 12 Finally, NBS can also detect healthy infants who are carriers; routine disclosure of this information can identify reproductive risks in parents and future adults.
Where the traditional outcome of NBS is not assured, the opportunity to acquire reproductive risk information may assume greater prominence, increasing the need to reconsider the relevance of parental discretion in pursuing such benefits. To this end, we turned to guidance from complementary paradigms – preconception (PCS) (community-based screening of at-risk groups) and prenatal screening (PNS) programmes – where the pursuit of reproductive risk information is generally the primary goal. We conducted a systematic review of relevant policies and position papers on PCS, PNS and NBS guidelines to examine: how guidelines construed generating reproductive risk information as a potential benefit of screening; and what conditions were seen to support the realization of this benefit.
Methods
Data sources
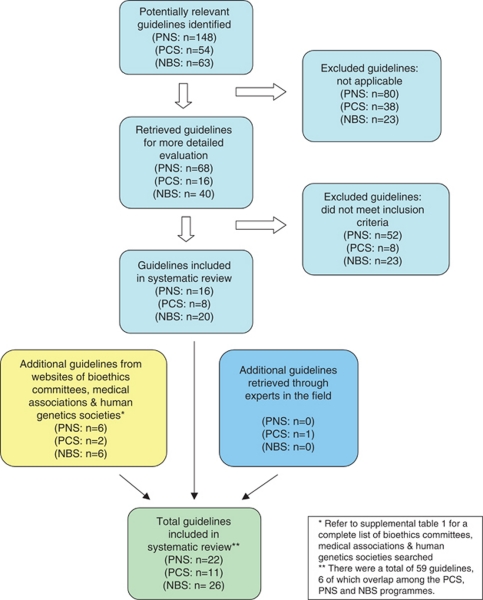
In accordance with the core principles of systematic review methodology,13 we conducted a review of relevant guidelines using HUMGEN (a database of laws and policies related to human genetics, which uses other databases, including PubMed, Google Scholar and others –www.humgen.org/int /_ressources/Method_en.pdf) (Figure 1). In addition, we searched websites of key organizations catalogued in HumGen, including the WHO, UNESCO, Council of Europe, national bioethics committees, human genetics societies and national medical associations (Supplementary Table 1). Additional guidelines were obtained from experts in the field. Our review included international and regional governmental and nongovernmental health organizations. In addition, guidance from national organizations limited to Europe, North America, the United Kingdom and Australasia were included, to represent jurisdictions that shared similar health-care and public health infrastructures. We searched the databases and websites using the following search terms: ‘preconception' [or] ‘reproductive' [or] ‘pre-pregnancy' [and] ‘genetic screening' [or] ‘screening' [or] ‘testing' ‘prenatal' [or] ‘pregnancy' [and] ‘genetic screening' [or] ‘screening' [or] ‘testing' and ‘newborn' [or] ‘neonatal' [or] ‘neonate' [and] ‘genetic screening' [or] ‘screening' [or] ‘testing'.
Figure 1.
Study selection.
Study selection
Policies were eligible for inclusion in our review if they were available position papers, reports or if they contained guidelines or statements produced by international, national and regional governmental and nongovernmental health organizations, bioethics committees or professional associations that explicitly addressed (1) newborn screening, (2) preconception screening or (3) prenatal screening. Only guidelines written in English or translated into English from relevant organizations were eligible for inclusion.
We excluded guidelines that did not explicitly include a statement that described the goals or purpose of the screening programme and that were published before 1996. This date restriction reflected the time period during which most relevant guidelines were produced. Guidelines focused on prenatal diagnosis, and those emanating from subnational organizations (eg, provinces or states) were also excluded. Finally, guidelines that focused on technical, organizational, laboratory or cost issues were ineligible. Any uncertainties regarding inclusion were discussed and agreed upon by 2–3 members of the team.
Data extraction and synthesis
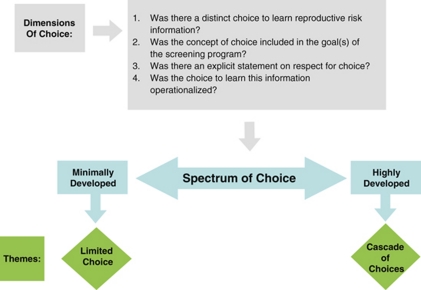
We used a qualitative content analytical approach to examine selected guidelines, drawing on the principles of qualitative description14 and constant comparison.15 First, guidelines were examined for an explicit description of the goals and benefits of the respective screening programme, noting the ways in which the generation of reproductive risk information was construed. The guidelines were then examined for an explicit description of how these benefits were to be realized. Specifically, we noted whether the screening programme was to be voluntary and whether informed consent was required. Focusing first on selected PCS and PNS guidelines, we developed an analytical framework describing the conditions or processes considered to support the pursuit of reproductive benefit. The analytical framework (Figure 2) included four dimensions of choice, which were then used to distil guidelines into a two-part spectrum with regard to the orientation towards voluntarism, from highly to minimally developed. Finally, this framework was used to examine the NBS guidelines, to identify similarities and differences in their orientation towards voluntarism.
Figure 2.
Analytical framework.
Results
We retrieved a total of 59 guidelines (Table 1) from 31 different organizations (six guidelines overlapped among PCS, PNS and NBS, yielding 53 unique guidelines). The guidelines originated from government-affiliated institutions (n=7), government advisory bodies (n=11), medical research agencies (n=3) and nongovernmental professional organizations (n=32). Altogether, 11 guidelines related to PCS, 22 on PNS and 26 pertained to NBS. The majority emanated from national organizations (n=49), with fewer from international (n=1) and supranational (n=3) bodies.
Table 1. Overview of programmatic guidance in reference to reproductive benefit and voluntarism.
| Theme | Screening programme guideline | ||
|---|---|---|---|
| Preconception screening | Prenatal screening | Newborn screening | |
| Recognize reproductive benefit? | All (11/11) | All (22/22) | Few (31−3/26) |
| Nature of voluntarism regarding reproductive benefit | |||
| 1. Cascade of Choices | 1. SOGC: 20064 2. NSGC: 20055 3. Australian Law Reform Commission: 20036 4. ACMG: 20017 5. Committee for Public Relations and Ethical Issues of the German Society of Human Genetics: 20058 6. NSGC: 19999 7. ACMG: 199810 8. ASHG: 200811 9. ACOG: 200512 | 1. New Zealand Government – Ministry of Health: 200713 2. Human Genetics Commission: 20061 3. NHS: 200514 4. Health Council of the Netherlands: 200415 5. French National Consultative Ethics Committee for Health and Life Sciences: 200316 6. NHS R&D HTA Programme: 199817 7. NHS: 200618 8. RCOG: 199719 9. RANZOG: 200720 10. ACMG: 200821 11. ACMG: 200822 12. SOGC: 199823 13. SOGC: 200724 14. NSGC: 200525 15. NIH: 199726 16. Swedish National Council on Medical Ethics: 200727 17. US Preventive Services Task Force: 199628 | 1. aHealth Council of the Netherlands: 20052 |
| 2. Limited choice | 1. NHGRI: 199729 2. US Preventive Services Task Force: 199628 | 1. NHGRI: 199729 2. NSGC: 20055 3. Swedish Council on Technology Assessment in Health Care: 200130 4. RANZCOG: 200631 5. German National Ethics Council: 200332 | 1. Human Genetics Commission: 20061 2. aCDC: 20043 |
| Other (NBS guidelines reviewed but did not mention reproductive benefit) | 1. aNHS: 200514 2. Association of State and Territorial Health Officers: 200533 3. aUK NBS Programme Centre: 200534 4. PSNZ: 200435 5. aHGSA: 200436 6. aACOG: 200737 7. AAP: 200138 8. aAssociation of Public Health Laboratories: 200239 9. CORN: 200040 10. aAAP: 200041 11. aAAP: 200042 12. aALRC: 20036 13. aNHS: 200643 14. ACMG: 200544 15. aAWHONN: 200545 16. ISNS: 200146 17. NIH: 200047 18. aNHS: 200618 19. aAAP: 200748 20. National Newborn Screening and Genetics Center: 200149 21. aNHS: 200850 22. AAP: 200751 23. US Preventive Services Task Force: 199628 | ||
Refers to guidelines that advocated voluntarism in pursuing the traditional clinical benefits of newborn screening.
For references see Supplementary Material.
Most preconception and prenatal guidelines – where generating reproductive risk information was a primary benefit of screening – required that individuals be given a ‘cascade of choices' regarding reproductive risk information. By contrast, guidance for NBS infrequently attended to the potential for reproductive benefits as a primary or secondary goal. Further, most guidelines that acknowledged such benefits construed voluntarism narrowly, offering ‘limited choices' that did not attend to the specific choices arising from the pursuit of reproductive risk information.
Recognizing reproductive benefit
It is not surprising that a sharp contrast was apparent in how PCS and PNS guidance approached reproductive benefit when compared with how this benefit was considered in NBS policies. All PCS and PNS guidelines stated that the pursuit of reproductive risk information was the primary benefit of screening (PCS: n=11 of 11, PNS: n=22 of 22), whereas few (n=3) NBS guidelines identified reproductive benefit as an intended or unintended benefit of screening (Table 1).
For example, in its statement entitled ‘Essentially Yours: The Protection of Human Genetic Information in Australia'16 the Australian Law Reform Commission clearly stipulated that the purpose of PCS is ‘to alert individuals to their carrier status so that they are able to make informed decisions about reproduction' (note 24.30). Similarly, in the PNS context, guidelines explicitly oriented the programmes towards the immediate benefit of this risk information.
Conversely, few of the NBS guidelines (n=3 of 26) identified the generation of reproductive information as a benefit of NBS: it was seen as a primary aim in one guideline and as an ‘indirect' benefit to the infant and family in the other two. The Human Genetics Commission's statement on ‘Making Babies: Reproductive Decisions and Genetic Technologies'17 acknowledged reproductive benefit as an indirect and limited end, stating that newborn screens are ‘not simply relevant to the care of the child involved. But because they may lead to the diagnosis of a genetic condition they can have implications for future reproductive decision-making by parents' (pp 41). However, the other two guidelines pointed to ways in which reproductive benefits may achieve elevated significance in this context. In their statement on ‘Newborn Screening',18 the Health Council of the Netherlands acknowledged both the existence of an ‘indirect' reproductive benefit, and the potential for this benefit to assume primary importance in cases in which the typical goal of clinical benefit cannot be achieved:
The identification of patients with a hereditary disorder also brings to light parent carriers. This discovery allows future family planning choices to be made in families with what are usually serious hereditary disorders… The opportunity to make choices is a benefit for the family, and sometimes also for the newborn child… The indirect benefits for the patient and the benefits for the family may result in screening being contemplated where there is little if any direct benefit to be had. Patients' organisations have taken the view that screening should not automatically be ruled out even if no treatment is available. The Committee shares this opinion. (pp 29)
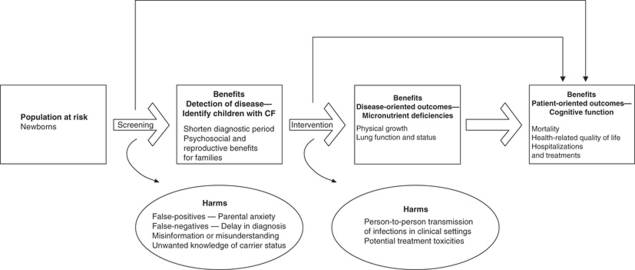
Importantly, the Center for Disease Control's recommendations with regard to NBS for cystic fibrosis (CF)19 included ‘reproductive benefits to families' as one of the primary benefits, alongside the other primary goals of disease detection and avoidance of the diagnostic odyssey, as illustrated in Figure 3:
The benefits of screening flow from early, asymptomatic detection and can be classified in terms of health benefits to the affected person and psychosocial benefits to persons and families… Another potential benefit to parents from a diagnosis of CF by newborn screening is the ability to make informed decisions related to further childbearing, because the diagnosis might occur 1 year earlier on average compared with conventional diagnosis (0.5 and 14.5 months, respectively). (pp 10 and 23)
Figure 3.
Potential benefits and harms of newborn screening for cystic fibrosis, as identified by Grosse and colleagues19 (figure reproduced with permission).
All PCS and PNS guidelines positioned the generation of reproductive risk information as the primary goal and benefit of screening programmes, and did so uniformly. Reproductive benefit in NBS guidelines, by contrast, was noted in only three guidelines. However, although clearly framed as ‘indirect' to the main goal of identifying treatable disorders in two of these, the potential of this indirect goal to assume primary significance was acknowledged; further, it was seen as a primary benefit in the other guideline.
Achieving the benefits of screening
All PCS and PNS guidelines emphasized voluntarism as the means of achieving the benefits of screening, requiring that individuals be given the choice to learn of their reproductive risk information (PCS: n=11 of 11; PNS: n=22 of 22). It is interesting that 15 out of 26 of the NBS guidelines advocated voluntarism in pursuing the traditional clinical benefits of screening; however, only one of the three NBS policies that recognized reproductive benefit highlighted voluntarism as the means of pursuing this specific benefit. Consequently, we identified a spectrum through which the choice to pursue reproductive benefit was described across the three screening programmes, with some guidelines emphasizing a fulsome ‘cascade of choices' and others construing voluntarism more narrowly as a set of ‘limited choices' (Tables 1 and 2).
Table 2. Example statements regarding voluntarism among the screening programmes grouped by theme.
| Nature of voluntarism | Programme guideline | ||
|---|---|---|---|
| Preconception screening | Prenatal screening | Newborn screening | |
| 1. Cascade of choices | Society of Obstetricians and Gynecologists of Canada (2007): ‘The purpose of population-based carrier screening is to identify carrier couples who are at risk of having an affected child and offer them counselling and the option of prenatal diagnosis.' (pp 330) National Society of Genetic Counselors (2005): ‘Individuals/couples considering screening should be provided with accurate, balanced information about the condition for which screening is being offered. They should be informed of the specificity, sensitivity, accuracy, risks, benefits and limitations of the screening tests offered and of any follow-up diagnostic tests as well as their reproductive options given a positive diagnostic test result.' American College of Medical Geneticists (2001): ‘To identify carrier couples with a one in four risk for affected offspring with each pregnancy and offer them genetic counseling and various reproductive options, including prenatal diagnosis' (pp 149) | New Zealand Government – Ministry of Health (2007): ‘If antenatal screening for Down's syndrome is to continue in New Zealand it should be: (a) universally offered to all pregnant women (b) accompanied by high-quality, balanced, easily understood information in an accessible range of formats for practitioners and consumers (c) conditional on informed consent (d) based on voluntary participation at each stage of the screening and diagnostic pathway (e) based on unconditional acceptance of, and support for, the choices made by women as a result of screening and diagnostic tests based on voluntary participation at each stage of the screening and diagnostic pathway' (pp 3) NHS (2006): ‘To offer* timely antenatal sickle cell and thalassaemia screening to all women (and couples) to facilitate informed decision-making (the offer* includes: the offer of, uptake of, and reporting of results of prenatal diagnosis and any subsequent action by the end of 12 weeks of pregnancy)… (pp 18)' … ‘The aim of this part of the Programme is to offer reproductive choice, including the offer of termination of pregnancy, if chosen… All women and their partners should receive information about antenatal screening options as early as possible in pregnancy, before they are asked to make any screening decision.' (pp 44) American College of Medical Geneticists (2008): ‘Such diagnostic testing should be made available if requested after appropriate counseling including risks and benefits. Likewise, women who do not want any further information regarding chromosomal status of their fetus should not be required to undergo any further testing or screening. Screening provides such an option for those women who would like to further refine their risk before deciding whether or not to undergo invasive testing.' (pp 74) | Health Council of the Netherlands (2005): ‘Participation in screening is voluntary and participants should be properly informed. The parents' informed consent is requested. They act on behalf of and in the interests of the newborns.' (pp 12) ‘Parents ought therefore to be given the option of forgoing information about carrier status at the point in time when the information is provided (during pregnancy, as advocated in Section 4.4.3). If, however, they should ask for carrier screening after receiving the information, then this request can be satisfied if this is medically indicated (owing to a family history of the disorder in question or, in the case of hemoglobinopathy, the geographical origin of the affected individuals)' (pp 78) |
| 2. Limited choice | National Human Genome Research Institute (1997): ‘Respect for an individual's/couples' beliefs and values concerning tests undertaken for assisting reproductive decisions is of paramount importance and can best be maintained by a nondirective stance. One way of ensuring that a nondirective stance is taken and that parents' decisions are autonomous, is through requiring informed consent.' US Preventive Services Task Force (1996): ‘Informed reproductive choices by receiving genetic counseling' | Swedish Council on Technology Assessment in Health Care (2001): ‘The use of available tests must be driven by the wishes of the parents. Hence it is important to provide parents with evidence-based, comprehensive information on the expected consequences of various tests and advise them that other disorders might also be identified.' The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (2006): ‘As with any test or procedure, these investigations should only be undertaken with the informed consent of the patient after adequate and appropriate counseling as to the implications, limitations and consequences of such investigation.' (pp 1) National Society of Genetic Counsellors (2005): The NSGC supports an individual's right to full disclosure of all appropriate medical options regarding reproductive testing and management of genetic diseases and birth defects. It is the care provider's responsibility to provide effective communication of all available options and to obtain informed consent for procedures involving risk to the individual or fetus.' (section: ‘informed choice') | Centre for Disease Control (2005): ‘Documentation of consent might not be necessary. The focus should be on providing thorough, easily understood information to parents about screening for CF and other conditions, especially before delivery, to reduce misunderstanding and provide parents with an opportunity to make informed choices, consistent with state laws.' (pp 28) ‘States are recommended to work with each other and with professional organizations and federal agencies to develop approaches to provide newborn screening information to parents during the prenatal and perinatal periods on all conditions, including CF, to facilitate informed choices and appropriate responses to positive screen results.' (pp 29) |
Cascade of choices
The majority of PCS (9 of 11) and PNS (16 of 22) guidelines clearly presented a cascade of choices in pursuing reproductive benefit. Voluntarism within these guidelines involved a set of nested decisions, each one preceding and enabling action on the other, by sequentially referring to the following: (i) the choice ‘to pursue' reproductive risk information; (ii) the choice ‘to know' diagnostic information in light of the risk information received; and where relevant; (iii) the choice ‘to act,' specifically, whether to continue a pregnancy. The New Zealand's Ministry of Health PNS guideline20 draws a clear distinction between the elements in this cascade of choices: (i) to pursue reproductive risk information (‘who choose to have this information'); (ii) ‘to make informed decisions about whether to have diagnostic testing' and, (iii) ‘to make informed decisions about whether to continue or terminate the pregnancy' (pp 17). Importantly, these distinct choices are stipulated as part of the goals of the programme. Similarly, the National Society of Genetic Counsellors' ‘Preconception/Prenatal Genetic Screening' guidelines21 emphasize this cascade of choices:
Individuals/couples considering screening should be provided with accurate, balanced information about the condition for which screening is being offered. They should be informed of the specificity, sensitivity, accuracy, risks, benefits and limitations of the screening tests offered and of any follow-up diagnostic tests as well as their reproductive options given a positive diagnostic test result (pp 3).
Guidelines offering a cascade of choices emphasized a high degree of respect for individuals' sequential choices in pursuing reproductive benefit. For example, the overall principle within New Zealand's statement on PNS20 focused on an ‘unconditional acceptance and support' for women's choices ‘at each stage of the screening and diagnostic pathway' (pp 3). Within the United Kingdom's Human Genetics Commission guidelines in reference to PNS,17 the choice to participate in screening is emphasized in their discussion on the ‘ethos of offering screening.' They stressed the importance of explaining the ‘aims of screening' before booking the screening appointment to ensure that the ‘offer of screening' was seen as a ‘real option' rather than as a ‘default option' so as ‘to minimize any sense of guilt or attribution of blame for a decision not to participate' (pp 12).
In the NBS context where reproductive benefit was recognized as a discrete benefit in only three guidelines,17, 18, 19 the Health Council of the Netherlands18 published the only policy that advanced a cascade of choices with regard to reproductive benefit. Although reproductive benefit was framed as an ‘indirect' benefit, they acknowledged that:
Special attention needs to be paid to providing information about the possibility of screening revealing that a newborn is a carrier. This practically always means that one or both parents are also carriers. As with parents of an affected child, if required, adequate information must also be available on what being a carrier entails and on the disorder concerned. (pp 16)
They required that a choice be offered as to whether parents want to learn incidentally generated reproductive risk information (ie, child's carrier status), and restricted the pursuit of this benefit to ‘medically indicated' cases:
Parents ought therefore to be given the option of forgoing information about carrier status at the point in time when the information [about NBS in general] is provided (during pregnancy, as advocated in Section 4.4.3). If, however, they should ask for carrier screening after receiving the information, then this request can be satisfied if this is medically indicated (owing to a family history of the disorder in question or, in the case of hemoglobinopathy, the geographical origin of the affected individuals). (pp 78)
Guidelines in this category paid significant attention to the voluntary pursuit of reproductive risk information, as well as to the respect for individuals' choices. They offered a distinct choice to learn reproductive risk information and distinguished between the sequential or nested nature of the choices inherent in pursuing such information.
Limited choice
Of the guidelines recognizing reproductive benefit, a few of the PCS (2 of 11) and PNS (6 of 22) policies presented voluntarism as a limited array of choices; where voluntarism was construed more narrowly, guidelines paid little attention to the sequential nature of choices afforded to individuals in pursuing reproductive risk information. Similarly, two of the remaining three NBS guidelines that identified the possibility of reproductive benefit did not reflect on voluntarism at all, and importantly, the choices specific to reproductive risk information were entirely absent.
Several of the PCS and PNS policies presented limited choices in the pursuit of reproductive benefit. The PCS guideline by the US Preventive Services Task Force,22 for example, highlighted the need for education and counselling without an explicit distinction between the types of choices offered (‘informed reproductive choices by receiving genetic counseling'). Among others, voluntarism was referred to abstractly or was otherwise absent. For example, in the PNS guideline prepared by the Royal Australian and New Zealand College of Obstetricians and Gynaecologists,23 the condition to achieve voluntarism seems to refer to one point in time, rather than to a process encompassing several stages:
As with any test or procedure, these investigations should only be undertaken with the informed consent of the patient after adequate and appropriate counseling as to the implications, limitations and consequences of such investigation.
The choice regarding reproductive risk information was largely ignored in the two remaining NBS guidelines that recognize reproductive benefit.17, 19 The CDC's19 guidelines on NBS for CF advocated voluntarism only with respect to the choice to participate in the NBS programme as a whole, without attention to the specific choices attendant on receiving reproductive risk information. It asserted:
Documentation of consent might not be necessary. The focus should be on providing thorough, easily understood information to parents about screening for CF and other conditions, especially before delivery, to reduce misunderstanding and provide parents with an opportunity to make informed choices, consistent with state laws. (pp 28)
The Human Genetics Commission's recommendations17 did not mention voluntarism in relation to either participating in NBS programmes generally or pursing reproductive risk information through such programmes, more specifically.
Guidelines in this category reflected only partially on the conditions supporting choice in the receipt of reproductive risk information. Where voluntarism was addressed, it was characterized by broad statements gesturing to the need for choice in achieving the benefits of screening, but without attention to an initial choice to receive reproductive risk information, nor to the sequential, nested choices of acting on that information. Importantly, of the two remaining NBS guidelines that identified the potential for reproductive benefits, only one supported any form of voluntarism. However, the voluntarism advocated was solely for participating in screening as a whole and not for the pursuit of reproductive risk information; in the final case, no form of voluntarism was identified.
Discussion
The diversity of NBS programmes along with rapid technological advances requires an assessment of the direction of NBS programmes. These issues are not without controversy and there is considerable international dissensus on the appropriate scope of NBS panels and the types of discretion to be afforded to parents.24, 25, 26, 27 Central to these debates is the balance of risks and benefits, which also remains equivocal. Although the potential risks – namely, psychosocial harms from false-positive results,28 overmedicalization,29, 30 misattributed paternity,31 stigma and discrimination32 – have remained the same, notions of benefit have evolved. Benefits, as argued by some, extend beyond the strictly medical model to include benefits previously considered secondary, such as early intervention, avoidance of the ‘diagnostic odyssey' and guidance for reproductive decision making.3, 5, 6, 7, 27 Indeed, NBS practice is changing, as an increasing number of jurisdictions have embraced the potential value of these broader benefits and have expanded their panels accordingly.27, 33, 34 Guidelines, however, have not evolved in tandem to reflect on the realization of these expanded benefits, nor on the choices regarding the pursuit of reproductive benefits.
Given that NBS typically operates as a mandatory or implied consent programme, reproductive risk information becomes packaged into this programme, effectively requiring parents to receive reproductive risk information,35 which is at odds with the principles of voluntarism and nondirectiveness underpinning the PCS and PNS programmes.36 Automatic disclosure of this risk information in the context of NBS is surely appropriate in cases in which infants can be expected to receive health benefits. Yet, it might be seen to violate parents' autonomy,35 in cases in which the benefit of reproductive risk information achieves particular prominence. This may occur in three specific ways including: (1) screening for rare conditions (eg, Duchenne muscular dystrophy) that do not have accepted treatment, yet early diagnosis and assisting reproductive decision making are considered important benefits;10, 11, 12 (2) expanded NBS panels that identify conditions without clear evidence of clinical benefit for affected infants,33 wherein the benefits of acquiring reproductive risk information are assuming greater, if not equivalent, importance, blurring the lines between primary and secondary benefits;9 and (3) incidental results (eg, carrier status) that are clinically benign for the infant, yet (potentially) immediately useful to his or her parents in planning future pregnancies. In these scenarios, the significance and relevance of this information for reproductive planning raises anew questions on how to pursue reproductive benefit.
The PCS and PNS guidelines offer direction for considering the realization of reproductive benefit through NBS. These guidelines are sensitive to the issues inherent in screening for the purpose of reproductive benefit. In addressing such a benefit, PCS and PNS guidance seeks to mobilize people's capacity to make sequential choices, and typically emphasizes the importance of a fulsome ‘cascade of choices' to ensure a well-informed decision-making process, which differentiates between the choice to pursue reproductive risk information, the choice to know how this risk information pertains to a specific pregnancy and the choice to act on a diagnosis. By contrast, although many NBS guidelines support voluntarism regarding participation in the programme as a whole, only two of those that identified the potential for reproductive benefits supported any form of voluntarism. Whereas one guideline supported a cascade of choices, in another, the pursuit of reproductive risk information was collapsed into the choice to participate in screening as a whole, thus highlighting challenges in the ethical pursuit of reproductive benefit through NBS.
Although it is widely agreed that the fundamental goal of NBS is to benefit the infant,37 and further, that the ethical norm of respect for persons necessitates that infants not be treated as a means to an end, debate continues as to whether the fact that NBS can inform parents about their reproductive risks is, in itself, a justification for an expanded panel or for a routine disclosure of incidental results.4, 5, 9, 38, 39 Indeed, an inherent tension exists between the obligations of serving the primary health interests of children, respecting autonomy and reducing potential harms in view of the current routine provision of most NBS programmes. The automatic disclosure of reproductive risk information in the absence of a fulsome consent process defies each of these obligations equally.7, 40, 41, 42 This is especially salient in light of recent evidence suggesting that the broader benefits encouraged by expanded NBS programmes may effect subtle and complex familial and social harms.43 PCS and PNS programmes, conversely, provide alternative methods of achieving reproductive benefits without imposing the myriad of moral burdens identified here. Further, such programmes offer a more fulsome cascade of choices to parents as illustrated by this review.
Limitations
Typical of systematic reviews of literature, our review has omitted guidance that may have provided additional insight into how to pursue reproductive benefit in the context of NBS. Although beyond the scope of the paper, guidelines produced by consumer groups and disease-centred organizations were omitted because of our focus on guidance produced by professional, health organizations or committees. The deliberate exclusion of guidelines pertaining to prenatal diagnosis, for the sake of direct comparison, may have also limited the scope of insights gleaned with regard to the extent of parental discretion afforded in such programmes. Finally, our interpretation of the nature of consent presented in these guidelines offers a typology that was restricted by the lens we adopted to answer our particular research question. The interpretations and typology are thus tentative and we are unable to immediately generalize to all guidelines related to these programmes.
Future research
Recognizing that guidelines are meant to be reflections on the appropriate use of particular health technologies, it is unknown how such guidance actually affects those it is meant to serve. Further research on stakeholders' perspectives on these expanded notions of benefit may illuminate their opinions on the risk/benefit ratio of NBS and when parental discretion may be important. Appropriate consent models should also be explored with stakeholders, noting both preferences and capacity for its provision.44 Finally, a fulsome study of stakeholders' informed preferences for PCS or prenatal versus NBS approaches in pursuing reproductive benefit is also warranted. Ultimately, further discussion and analysis is required to address the implications of providing reproductive risk information as a primary benefit of a population-screening programme. Salient questions arise, including whether consent for and/or education on pursuing reproductive risk information is included in the context of NBS; who should be responsible for this process; and who should ensure that the future-adult infant is informed of his or her carrier status. Further, once such a benefit is pursued as part of a population intervention, should parents and other family members (eg, minors) be invited to undergo carrier testing, and how should cascade testing be carried out?
Conclusions
This review suggests that guidance from prenatal and preconception contexts identifies a coherent framework for the pursuit of reproductive benefits through population screening. Thus, when NBS no longer serves the primary goal of clinical benefit for affected infants (eg, in cases in which infant carrier results are generated, or in the absence of demonstrated clinical benefit for an affected infant), it should seek to introduce a ‘cascade of choices' in pursuing reproductive benefits. Alternatively, achieving reproductive benefit may be best pursued through PCS and PNS.
Acknowledgments
We acknowledge the financial support provided for the following individuals: Yvonne Bombard was supported by a Postdoctoral fellowship from Apogee-Net, a network funded by the Canadian Institutes of Health Research (CIHR); Fiona A Miller is supported by a New Investigator Award from the Institute of Health Services and Policy Research of the CIHR (FRN # 80495); Robin Z Hayeems is supported by a CADRE Postdoctoral Fellowship from the Canadian Institutes of Health Research and the Canadian Health Services Research Foundation; Bartha Maria Knoppers and Denise Avard are supported by Genome Quebec and Genome Canada.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Grosse SD, Boyle CA, Kenneson A, Khoury MJ, Wilfond BS. From Public Health Emergency to Public Health Service: the implications of evolving criteria for newborn screening panels. Pediatrics. 2006;117:923–929. doi: 10.1542/peds.2005-0553. [DOI] [PubMed] [Google Scholar]
- American College of Medical Genetics . Newborn Screening: Toward a Uniform Screening Panel and System. Bethesda: American College of Medical Genetics; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DB, Skinner D, Warren SF. Newborn screening for developmental disabilities: reframing presumptive benefit. Am J Public Health. 2005;95:1889–1893. doi: 10.2105/AJPH.2004.051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D, van Dyck PC. A vision of the future of newborn screening. Pediatrics. 2006;117:350–354. doi: 10.1542/peds.2005-2633O. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Beskow LM, Davis AM, Skinner D. Changing perspectives on the benefits of newborn screening. Ment Retard Dev Disabil. 2006;12:10. doi: 10.1002/mrdd.20119. [DOI] [PubMed] [Google Scholar]
- Laird L, Dezateux C, Anionwu EN. Neonatal screening for sickle cell disorders: what about the carrier infants. Br Med J. 1996;313:407–411. doi: 10.1136/bmj.313.7054.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews LB, Fullarton JE, Holtzman NA, Motulsky AG. Assessing Genetic Risks: Implications for Health and Social Policy. Washington: National Academy Press; 1994. [PubMed] [Google Scholar]
- Green NS, Dolan SM, Murray TH. Newborn screening: complexities in universal genetic testing. Am J Public Health. 2006;96:1955–1959. doi: 10.2105/AJPH.2005.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombard Y, Miller FA, Hayeems RZ, et al. The expansion of newborn screening: is reproductive benefit an appropriate pursuit. Nat Rev Genet. 2009;10:666–667. doi: 10.1038/nrg2666. [DOI] [PubMed] [Google Scholar]
- Ross LF. Screening for conditions that do not meet the Wilson and Jungner criteria: the case of Duchenne muscular dystrophy. Am J Med Genet A. 2006;140:914–922. doi: 10.1002/ajmg.a.31165. [DOI] [PubMed] [Google Scholar]
- Bradley DM, Parsons EP, Clarke AJ. Experience with screening newborns for Duchenne muscular dystrophy in Wales. BMJ. 1993;306:357–360. doi: 10.1136/bmj.306.6874.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuerbrandt G, Lundin A, Lovgren T, Mortier W. Screening for Duchenne muscular dystrophy: an improved screening test for creatine kinase and its application in an infant screening program. Muscle Nerve. 1986;9:11–23. doi: 10.1002/mus.880090103. [DOI] [PubMed] [Google Scholar]
- Lavis J, Davies H, Oxman A, Denis JL, Golden-Biddle K, Ferlie E. Towards systematic reviews that inform health care management and policy-making. J Health Serv Res Policy. 2005;10 (Suppl 1:35–48. doi: 10.1258/1355819054308549. [DOI] [PubMed] [Google Scholar]
- Sandelowski M. Whatever happened to qualitative description. Res Nurs Health. 2000;23:334–340. doi: 10.1002/1098-240x(200008)23:4<334::aid-nur9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Strauss A, Corbin J.Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory2nd edn.Thousand Oaks: Sage Publications; 1998 [Google Scholar]
- Australian Law Reform Commission . Essentially Yours: The Protection of Human Genetic Information in Australia. Sydney: Australian Law Reform Commission; 2003. [PubMed] [Google Scholar]
- Human Genetics Commission . Making Babies: Reproductive Decisions and Genetic Technologies. London: Human Genetics Commission; 2006. [Google Scholar]
- Health Council of the Netherlands . Neonatal Screening. The Hague: Health Council of the Netherlands; 2005. [Google Scholar]
- Scott D, Grosse PD, Coleen A, et al. Newborn screening for cystic fibrosis: evaluation of benefits and risks and recommendations for state newborn screening programs. Morb Mortal Wkly Rep. 2004;53:1–36. [PubMed] [Google Scholar]
- New Zealand Ministry of Health . Antenatal Down Syndrome Screening in New Zealand. Wellington: New Zealand Ministry of Health; 2007. [Google Scholar]
- National Society of Genetic Counselors . Preconception/Prenatal Genetic Screening. Chicago: National Society of Genetic Counselors; 2005. [Google Scholar]
- U.S. Preventative Services Task Force . Congenital Disorders-Screening for Hemaglobinopathies. Rockville: Agency for Healthcare Research and Quality; 1996. [Google Scholar]
- The Royal Australian and New Zealand College of Obstetricians and Gynaecologists . Antenatal Screening Tests. East Melbourne: The Royal Australian and New Zealand College of Obstetricians and Gynaecologists; 2006. [Google Scholar]
- Mandl KD, Feit S, Larson C, Kohane IS. Newborn screening program practices in the United States: notification, research, and consent. Pediatrics. 2002;109:269–273. doi: 10.1542/peds.109.2.269. [DOI] [PubMed] [Google Scholar]
- Hiller EH, Landenburger G, Natowicz MR. Public participation in medical policy-making and the status of consumer autonomy: the example of newborn-screening programs in the United States. Am J Public Health. 1997;87:1280–1288. doi: 10.2105/ajph.87.8.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ED, Ross LF. Incorporating newborn screening into prenatal care. Am J Obstet Gynecol. 2004;190:1. doi: 10.1016/j.ajog.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Pollitt RJ. Introducing new screens: why are we all doing different things. J Inherit Metab Dis. 2007;30:423–429. doi: 10.1007/s10545-007-0647-2. [DOI] [PubMed] [Google Scholar]
- Hewlett J, Waisbren S. A review of the psychosocial effects of false-positive results on parents and current communication practices in newborn screening. J Inherit Metab Dis. 2006;29:677–682. doi: 10.1007/s10545-006-0381-1. [DOI] [PubMed] [Google Scholar]
- Marteau TM, van Duijn M, Ellis I. Effects of genetic screening on perceptions of health: a pilot study. J Med Genet. 1992;29:24–26. doi: 10.1136/jmg.29.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FA, Paynter M, Hayeems RZ, et al. Understanding sickle cell carrier status identified through newborn screening: a qualitative study. Eur J Hum Genet. 2009;18:303–308. doi: 10.1038/ejhg.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen A, Parker M. Revealing false paternity: some ethical considerations. The Lancet. 2001;357:1033–1035. doi: 10.1016/S0140-6736(00)04240-9. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Skinner D, Davis AM, Whitmarsh I, Powell C. Ethical, legal, and social concerns about expanded newborn screening: fragile X syndrome as a prototype for emerging issues. Pediatrics. 2008;121:e693–e704. doi: 10.1542/peds.2007-0820. [DOI] [PubMed] [Google Scholar]
- Watson MS, Mann MY, Lloyd-Puryear MA, Rinaldo P, Howell RR, American College of Medical Genetics Newborn Screening Expert G Newborn screening: toward a uniform screening panel and system – executive summary. Pediatrics. 2006;117:S296–S307. doi: 10.1542/peds.2005-2633I. [DOI] [PubMed] [Google Scholar]
- Ontario Ministry of Health and Long Term Care McGuinty Government Expands Newborn Screening, 2006Accessed 3 March 2008.
- Miller FA, Robert JS, Hayeems RZ. Questioning the consensus: managing carrier status results generated by newborn screening. Am J Public Health. 2009;99:210. doi: 10.2105/AJPH.2008.136614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser FC. Genetic counseling. Am J Hum Genet. 1974;26:636–659. [PMC free article] [PubMed] [Google Scholar]
- Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. Geneva: World Health Organization; 1968. [Google Scholar]
- Miller FA, Robert JS, Hayeems RZ. Questioning the consensus: managing carrier status results generated by newborn screening. Am J Public Health. 2009;99:210–215. doi: 10.2105/AJPH.2008.136614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Council of the Netherlands . Screening: Between Hope and Hype. The Hague: Health Council of the Netherlands; 2008. [Google Scholar]
- Takala T, Hayry M.Genetic ignorance, moral obligations and social duties J Med Philos 200025107–113.discussion 114–120. [DOI] [PubMed] [Google Scholar]
- Borry P, Fryns JP, Schotsmans P, Dierickx K. Carrier testing in minors: a systematic review of guidelines and position papers. Eur J Hum Genet. 2006;14:133–138. doi: 10.1038/sj.ejhg.5201509. [DOI] [PubMed] [Google Scholar]
- European Society of Human Genetics Genetic testing in asymptomatic minors: recommendations of the European Society of Human Genetics. Eur J Hum Genet. 2009;17:720–721. doi: 10.1038/ejhg.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob R. Is my sick child healthy? Is my healthy child sick?: changing parental experiences of cystic fibrosis in the age of expanded newborn screening. Soc Sci Med. 2008;67:1056–1064. doi: 10.1016/j.socscimed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Miller FA, Hayeems RZ, Carroll JC, et al. Consent for newborn screening: the attitudes of health care providers Public Health Genomics 2009. e-pub ahead of print 22 September 2009. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.