Abstract
Kv4 potassium channel α subunits, Kv4.1, Kv4.2, and Kv4.3, determine some of the fundamental physiological properties of neurons in the CNS. Kv4 subunits are associated with auxiliary β-subunits, such as the potassium channel interacting proteins (KChIP1 – 4), which are thought to regulate the trafficking and gating of native Kv4 potassium channels. Intriguingly, KChIP1 is thought to show cell type-selective expression in GABA-ergic inhibitory interneurons, while other β-subunits (KChIP2–4) are associated with principal glutamatergic neurons. However, nothing is known about the expression of Kv4 family α- and β-subunits in specific interneurons populations in the BLA. Here, we have used immunofluorescence, co-immunoprecipitation, and Western Blotting to determine the relative expression of KChIP1 in the different interneuron subtypes within the BLA, and its co-localization with one or more of the Kv4 α subunits. We show that all three α-subunits of Kv4 potassium channel are found in rat BLA neurons, and that the immuno-reactivity of KChIP1 closely resembles that of Kv4.3. Indeed, Kv4.3 showed almost complete co-localization with KChIP1 in the soma and dendrites of a distinct subpopulation of BLA neurons. Dual-immunofluorescence studies revealed this to be in BLA interneurons immunoreactive for parvalbumin, cholecystokin-8, and somatostatin. Finally, co-immunoprecipitation studies showed that KChIP1 was associated with all three Kv4 α subunits. Together our results suggest that KChIP1 is selectively expressed in BLA interneurons where it may function to regulate the activity of A-type potassium channels. Hence, KChIP1 might be considered as a cell type-specific regulator of GABAergic inhibitory circuits in the BLA.
Keywords: BLA, KChIP1, parvalbumin, CCK-8, NPY, somatostatin
The basolateral amygdala (BLA) plays a critical role in the acquisition and expression of emotional learning and memory (LeDoux, 1992; Kim and Davis, 1993; Sigurdsson et al., 2007; Walker and Davis, 2002), and disturbances in the excitability of the BLA neurons can result in the pathogenesis of anxiety disorders, post-traumatic stress disorder (PTSD), phobias, and depression (Davis, 1992; Davis et al., 1994; Shekhar et al., 2005; Likhtik et al., 2008; Sah and Westbrook, 2008). The BLA contains two major neuronal classes; pyramidal projection neurons that utilize glutamate as a neurotransmitter, and non-pyramidal local circuit inhibitory interneurons, which utilize GABA as a neurotransmitter (McDonald and Mascagni, 2006). Although the interneuron population constitutes only 15–20% of the total population of BLA neurons, they play a critical role in controlling the overall excitability of the complex, including governing action potential generation, spike firing patterns, membrane potential oscillations, and dendritic calcium spikes (Cobb et al., 1995; Rainnie et al., 2006; Ehrlich et al., 2009).
At least four different subpopulations of interneurons have been described based on their expression of neuro-chemical markers such as calcium binding proteins [parvalbumin (PV), calretinin (CR), and calbindin (CB)], and neuropeptides [neuropeptide Y (NPY), cholecystokinin (CCK), vasoactive intestinal peptide (VIP), and somatostatin (SOM)] (McDonald and Mascagni, 2002). Significantly, these neurochemical markers identify functionally distinct subpopulations of interneurons in the BLA (McDonald and Mascagni, 2002; Levita et al., 2003), which can be differentiated by their electrophysiological properties (Rainnie et al., 2006; Woodruff and Sah, 2007; Jasnow et al., 2009). Since these electrophysiological properties are determined by the ion channels possessed by neurons, these subpopulations of interneurons would also be expected to show different ion channel expression profiles. Consistent with this hypothesis, immunohistochemical studies have shown that subunits of the Kv3 family of voltage-dependent potassium channel are differentially found in BLA interneurons (McDonald and Mascagni, 2006). Hence, both the Kv3.1 and Kv3.2 subunits are highly expressed in PV positive interneurons, whereas CCK interneurons express little, if any, of these subunits. Evidence suggests that these two Kv3 channel subunits are required for rapid action potential repolarization and high-frequency firing (Rudy and McBain, 2001). Consistent with this observation CCK interneurons in the BLA have significantly longer action potential duration than the PV subpopulation of interneurons (Jasnow et al., 2009).
Recent immunohistochemical studies have also revealed that GABAergic interneurons in the hippocampus (Menegola et al., 2008), striatum, and cortex (Rhodes et al., 2004) selectively express the Kv4.3 subunit of the Kv4 family of A-type voltage-gated potassium channels. Three different Kv4 channel α subunits (Kv4.1–Kv4.3) are expressed in the adult rat brain (Serodio and Rudy, 1998). Importantly, the α subunits are differentially distributed both regionally and compartmentally within neurons, where they function to tightly regulate the basic electrophysiological response properties of neurons (Covarrubias et al., 2008). Hence, the majority of Kv4 channel subunits are found in the soma and/or dendrites of central neurons. The A-type potassium channels that are composed of Kv4 channel subunits activate and inactivate rapidly in response to membrane depolarization and are known to regulate dendritic excitability, somatodendritic signal integration, and long-term potentiation (Birnbaum et al., 2004; Chen et al., 2006).
Importantly, native neuronal Kv4 potassium channels require auxiliary β-subunits to function, including potassium channel interacting proteins (KChIPs) and dipeptidyl-peptidase-like-proteins (DPPs). The KChIPs belong to the neuronal calcium sensor (NCS) family of calcium binding proteins, and bind to the cytoplasmic N-terminal of Kv4 family α subunits and regulate their biophysical properties (An et al., 2000). Hence, KChIPs facilitate Kv4 family subunit assembly, and modulate their surface density, inactivation kinetics, and rate of recovery from inactivation (An et al., 2000; Rhodes et al., 2004; Cui et al., 2008). Significantly, genetic deletion of Kv4.2 channel also eliminates expression of its associated KChIPs (Menegola and Trimmer, 2006), suggesting that the expression of KChIPs is necessitated on the functional expression of α-subunits of Kv4 potassium channel.
Four different KChIPs have been cloned: potassium channel interacting protein 1–4 (KChIP1–4). Importantly, KChIP1 and 2 interact only with the Kv4 family of voltage-gated potassium channels but not with other Kv alpha subunits (An et al., 2000). Moreover, in the hippocampus KChIP1 and Kv4.3 subunits are co-localized in the soma and dendrites of GABAergic interneurons (Menegola et al., 2008), suggesting that the expression of KChIP isoforms may be cell type specific. Indeed, KChIP1 has been recently proposed to be a specific modulator of the inhibitory GABAergic system (Xiong et al., 2009). The amygdala shows high levels of Kv4.3 subunit mRNA expression, whereas Kv4.2 mRNA expression is small or negligible (Serodio and Rudy, 1998). If the relationship between co-expression of Kv4.3 subunits and KChIP1 α subunits holds true for the amygdala, then KChIP1 may be preferentially expressed by interneurons in this region. However, nothing is known about the relative expression, cellular distribution, and/or association of KChIP1 and other KChIPs with Kv4 α subunits in the rat BLA. In this study, we used dual-immunofluorescence, Western Blot, and coimmunoprecipitation (co-IP) to examine the distribution and expression of KChIP1 in different interneuron subclasses, and its specific association with the three Kv4 potassium channel subunits.
EXPERIMENTAL PROCEDURES
Animal subjects
Experiments were performed on male, 45–60 days old male Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA, USA). All the procedures used were approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University and were in compliance with National Institutes of Health (NIH) guidelines for the care and use of laboratory animals.
Immunohistochemistry
Tissue processing
Dual-immunofluorescence experiments were performed on 4% paraformaldehyde-fixed rat brain sections derived from eight adult (45–60-days old) rats. Animals were first anaesthetized with sodium pentobarbital (100 mg/kg i.p. Butler-Schein Animal Health, Dublin, OH, USA), and then transcardially perfused with ice-cold 0.05 M phosphate buffer saline (PBS, pH 7.4) followed by 200–250 ml of 4% paraformaldehyde in PBS. Brains were removed and subsequently post-fixed in 4% paraformaldehyde for 2 h before being cryoprotected in 30% sucrose in PBS overnight at 4 °C. Coronal brain sections (50 μm) were cut on a Leica CM 3050S cryostat and stored at −20 °C in a cryoprotective medium consisting of 25% glycerol and 30% ethylene glycol in 0.05 M phosphate buffer until needed.
Antibodies
All antibodies used in our study are summarized in Table 1. The specificity of each of the primary antibodies has been verified using Western blots from brain tissue by the UC, Davis/NeuroMab Facility and also in our laboratory in BLA tissue extracts. All antibodies employed in immunofluorescence, Western blotting and co-IP experiments showed distinct bands in the immunoblots (see Results section 3.2. and Fig. 4).
Table 1.
Antibodies used in immunohistochemistry and Western Blotting
| Antibody | Host | Company | Dilution | Catalog number | Antigen |
|---|---|---|---|---|---|
| Kv4.1 0.6 mg/ml | Rabbit polyclonal | Sigma Aldrich (St. Louis, MO, USA) | 1:1000 | P-0118 | synthetic peptide from amino acids 538–550 of human Kv4.1 (C-terminal) |
| Kv4.2 1.03 mg/ml | Mouse monoclonal | UC Davis/NIH NeuroMab Facility (Davis, CA, USA) | 1:1000 | 75-016 Clone K57/1 | synthetic peptide from amino acids 209–225 (extracellular S1–S2 loop) of rat Kv4.2 |
| Antibodies Incorporated | |||||
| Kv4.2 0.60 mg/ml | Rabbit polyclonal | Alomone Labs (Jerusalem, Israel) | 1:1000 | APC-023 | peptide of amino acids 454–469 of rat Kv4.2 (C-terminal) |
| Kv4.3 1.04 mg/ml | Mouse monoclonal | UC, Davis/NIH NeuroMab Facility (Davis, CA, USA) | 1:1000 | 75-017 Clone K75/41 | fusion protein of amino acids 415–636 of rat Kv4.3 (C-terminal) |
| Antibodies Incorporated | |||||
| Kv4.3 0.80 mg/ml | Rabbit polyclonal | Alomone Labs (Jerusalem, Israel) | 1:1000 | APC-017 | peptide from amino acids 451–468 of human Kv4.3 (C-terminal) |
| Kv3.2 0.3 mg/mL | Rabbit polyclonal | Sigma Aldrich (St. Louis, MO, USA) | 1:500 | P9857 | synthetic peptide from amino acids 184–204 of rat Kv3.2 |
| KChIP1 1.05 mg/ml | Mouse monoclonal | UC, Davis/NIH NeuroMab Facility (Davis, CA, USA) | 1:1000 | 75-003 Clone K55/7 | full-length fusion proteins of amino acids 1–216 of rat KChIP1b |
| Antibodies Incorporated | |||||
| KChIP2 1.00 mg/mL | Mouse monoclonal | UC, Davis/NIH NeuroMab Facility (Davis, CA, USA) | 1:1000 | 75-004 Clone K60/73 | full-length fusion proteins of amino acids 1–252 of rat KChIP2b |
| Antibodies Incorporated | |||||
| KChIP3 1.02 mg/ml | Mouse monoclonal | UC, Davis/NIH NeuroMab Facility (Davis, CA, USA) | 1:1000 | 75-005 Clone 66/38 | full-length fusion proteins of amino acids 1–256 of rat KChIP3 |
| Antibodies Incorporated | |||||
| KChIP4 1.00 mg/ml | Mouse monoclonal | Abcam (Cambridge, MA, USA) | 1:1000 | ab57830 | full-length protein from amino acids 1–251 of human KChIP4 |
| PARV | Rabbit polyclonal | Swant (Bellinzona, Switzerland) | 1:1000 | PV-28 | rat muscle parvalbumin |
| SOM 1 mg/ml | Rabbit polyclonal | ImmunoStar (Hudson, Wi, USA) | 1:1000 | 20067 | somatostatin conjugated to Keyhole Limpet Hemocyanin with carbodiimide |
| CCK-8 61 mg/ml | Rabbit polyclonal | Sigma Aldrich (St. Louis, MO, USA) | 1:1000 | C 2581 | synthetic sulfated cholecystokinin (26–33) amide conjugated to KLH |
| CR | Goat polyclonal | Swant (Bellinzona, Switzerland) | 1:1000 | CG1 | human recombinant calretinin |
| NPY 1.46 mg/ml | Rabbit polyclonal | Chemicon-Millipore, Billerica, MA, USA) | 1:1000 | AB9608 | synthetic Neuropeptide Y |
| CaMKII α 1.00 mg/ml | Rabbit polyclonal | LifeSpan Biosciences, Seattle, WA, USA | 1:1000 | LS-B1178 | synthetic peptide for amino acids surrounding Serine 286 of human CAMK2A |
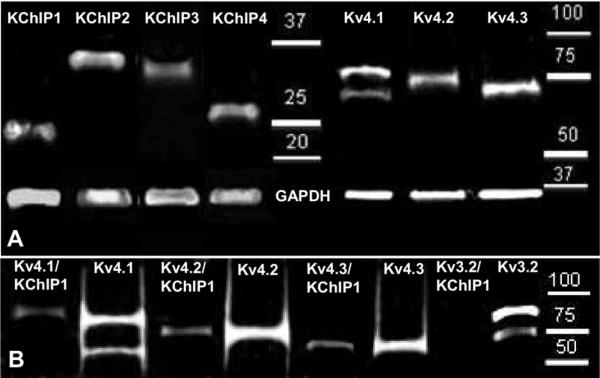
Fig. 4.
(A) Western Blot revealed presence of the distinct bands and confirmed expression of all four β-subunits of Kv4 potassium channel in the BLA. The strongest expression was observed for KChIP2 (molecular weight~37 kDa) and KChIP4 (~29 kDa), moderate for KChIP3 (~34 kDa) and the weakest for KChIP1 (~25 kDa). Distinct bands confirmed expression of all three α-subunits of the Kv4 potassium channel in the BLA: Kv4.1 (~60 and 87 kDa), Kv4.2 (~72 kDa), and Kv4.3 (~70 kDa) with the strongest expression observed for Kv4.3 subunit. (B) Co-precipitation experiment showed that all three α-subunits of the Kv4 potassium channel: Kv4.1, Kv4.2, and Kv4.3 were co-associated with KChIP1 in the BLA tissue extracts, while Kv3.2 potassium channel subunit did not co-precipitate with KChIP1.
Immunofluorescence experiment
To examine the relative expression of the Kv4 family α subunits and their β subunits KChIPs in the BLA, we performed immunofluorescence on free-floating serial sections of the rat BLA. Representative sections from bregma −2.12 mm to bregma −3.14 mm were rinsed 3× for 10 min in PBS, permeabilized with 0.5% Triton-X 100 in PBS, and incubated for 48 h at 4 °C with the primary antibody in 0.5% Triton-X/PBS solution. Sections were then rinsed 3× for 10 min in PBS and incubated at room temperature for 2 h with either Alexa-Fluor 488 goat anti-mouse IgG or Alexa-Fluor 568 goat anti-rabbit IgG or Alexa-Fluor 568 goat anti-mouse IgG (1:500, Molecular Probes, Invitrogen, Carlsbad, CA, USA) depending on the primary antibody's host. Sections were then rinsed 3× for 10 min in PBS, and 1× in phosphate buffer (PB), mounted on gelatin-coated glass slides and coverslipped using Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, CA, USA).
Dual-immunofluorescence experiment
To examine the possible co-localization of KChIP1 with Kv4.1, Kv4.2, and/or Kv4.3 we performed a dual-immunofluorescence staining procedure using mouse anti-KChIP1 and rabbit anti-Kv antibodies (see Table 1). We have tested the specificity of the rabbit Kv4 α-subunit antibodies with Western blots (Fig. 4B) and pre-incubation with antigen blocking peptides (Alomone Labs). In the latter procedure we pre-incubated all three primary rabbit antibodies with their respective antigen peptides for 1 h and then applied the antibodies to free-floating BLA sections and then followed immunofluorescence procedure outlined below. The Kv4.1 antigen peptide from Alomone Labs was also used to test the specificity of the Sigma Aldrich Kv4.1 antibody because both antibodies were raised in rabbit against exactly the same antigen (see Table 1). Pre-incubation of the rabbit primary antibodies with their respective blocking peptides completely abolished immunofluorescence for each α-subunit (data not shown).
A dual-immunofluorescence protocol was then used to determine the relative co-localization of KChIP1 with specific markers for each of the major subpopulations of BLA interneurons, namely PV, CR, cholecystokinin 8 (CCK-8), SOM, and NPY. To determine the relative expression of KChIP1 within the population of BLA principal neurons we performed additional experiments using a rabbit polyclonal antibody raised against calcium/calmodulin-dependent protein kinase α II (CaMKIIα). CaMKIIα is exclusively expressed in the BLA principal neurons and not in GABAergic interneurons (McDonald et al., 2002).
After 48 h incubation at 4 °C with a cocktail of the two primary antibodies, sections were rinsed in 3× for 10 min in PBS and subsequently incubated with a cocktail of two species-specific Alexa-conjugated secondary antibodies: Alexa-Fluor 488 goat anti-mouse IgG or Alexa-Fluor 568 donkey anti-mouse IgG and Alexa-Fluor 568 goat anti-rabbit IgG or Alexa-Fluor 488 donkey anti-goat IgG (1:500, Molecular Probes, Invitrogen, Carlsbad, CA, USA) for 2 h at room temperature. The sections were then rinsed and mounted as outlined above.
The stained sections were analyzed using an Olympus BX51 (Japan) microscope equipped with the appropriate fluorescein isothiocyanate (FITC) and Tetramethylrhodamine isothiocyanate (TRITC) excitation and emission fluorescent filter sets. Cell counting analysis was performed using Neurolucida Software (MBF Bioscience, Williston, VT, USA). Here, immunoreactive neurons for each specific marker were counted from representative sections of the entire BLA region outlined with the Neurolucida Software. Neurons from six to eight (n=6 – 8) sections containing the BLA (from 3 to 4 different animals for every marker stained) were counted and analyzed for dual immuno-labeling. Dual-immunofluorescence analysis was accomplished by focusing on neurons throughout the thickness of the section and by maintaining the same focal plan switching between filter sets to ascertain if the cell shows dual immunofluorescence. Confocal laser scanning microscopy was used to obtain high-resolution photomicrographs (63× magnification) using an Orca R2 cooled CCD camera (Hammamatsu, Bridgewater, NJ, USA) mounted on a Leica DM5500B microscope (Leica Mircosystems, Bannockburn, IL, USA).
Co-immunoprecipitation
To examine any physical association between KChIP1 and the individual Kv4 subunits we performed a co-IP study in tissue samples isolated from the BLA of 3 rats. Here, coronal brain slices (500 μm) were cut using a vibratome (Leica VT 1000S) and the entire BLA was dissected out from each slice and homogenized in 5 mM HEPES (pH=7.4) with 0.32 M sucrose and protease inhibitors. Following homogenization the protein concentration was determined using a bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford, IL, USA) and the samples were kept frozen at −80°C until required for the co-IP assay. Here, Nonidet P40 (USB Corporation, Cleveland, OH, USA) was added to 50 μg of sample protein to a final concentration of 1%, and then incubated by end-over-end mixing at 4 °C for 1 h to improve solubilization. Samples were then centrifuged at 4 °C (10,000g) for 10 min to remove insoluble material, and the supernatants collected and incubated overnight at 4 °C with 10 μl of protein A/G-Plus Agarose (sc-2003, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and 0.5 μl of KChIP1 antibody (1:200). On the following day, samples were washed 5× with 500 μl ice-cold homogenization buffer (as above), the precipitated proteins associated with the KChIP1 antibody were eluted from the beads via incubation with SDS-PAGE sample buffer. Precipitated samples were then probed in Western blots using primary rabbit antibodies against Kv4.1, Kv4.2, Kv4.3, and Kv3.2 potassium channel subunits. The Kv3.2 subunit was used as a negative control in our co-Ip experiment since this subunit is found at high levels in BLA interneurons (McDonald and Mascagni, 2006) but is not known to form a protein-protein complex with KChIP1. To avoid potential cross-reactivity issues between the secondary anti-mouse IgG antibody used in our Western blots (see below) and mouse IgG fragments potentially remaining in samples co-precipitated with the mouse KChIP1 antibody, all primary antibodies used to examine any Kv4 α-subunits co-precipitated with KChIP1 were raised in rabbit.
Western blotting
Western blots were used to examine the relative expression of the α- and β-subunits proteins in samples from the BLA of 4 rats (isolated as described above) or in samples from the co-IP experiment. In both cases, 25 μg of protein per sample was loaded onto polyacrylamide-SDS mini-gels (Bio-Rad, Hercules, CA, USA), separated electrophoretically, blotted onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA), and blocked for 1 h in blocking buffer containing 2% nonfat dry milk, 0.1% Tween 20, 0.05 M NaCl, and 1 M HEPES (pH 7.4). To examine the relative expression of all α- and β-subunits the membranes were incubated with the following primary antibodies: rabbit polyclonal anti-Kv4.1, and mouse monoclonal antibodies against Kv4.2, Kv4.3, KChIP1, KChIP2, KChIP3, and KChIP4 (see Table 1 for respective dilutions).
For the co-IP experiments 4 membranes were incubated separately overnight at 4 °C in the blocking buffer with the following primary antibodies: rabbit polyclonal anti-Kv4.1, rabbit polyclonal anti-Kv4.2 and Kv4.3, as well as rabbit polyclonal anti-Kv3.2 antibody.
On the following day, the membranes were incubated with HRP-labeled specific secondary antibody (peroxidase conjugated anti-rabbit IgG or peroxidase conjugated anti-mouse IgG, Vector Labs, 1:2000) for 1 h at room temperature. The proteins in the BLA samples as well as those co-precipitated with KChIP1 were detected using SuperSignal West Chemiluminescence (Pierce Biotechnology) and visualized with an Alpha Innotech Fluorochem imaging system (Alpha Innotech, San Leandro, CA, USA). After signal development, nitrocellulose membranes were stripped by incubating for 20 min with Restore Plus Western Blot Stripping Buffer (Thermo Scientific), washed with 0.05 M PBS and probed again with a mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primary antibody (10R-G109a, Fitzgerald Industries International INC, Concord, MA, USA 1:2000) as above. Finally, the relative integrated intensity values for each sample was analyzed using the Alpha Innotech Fluorochem imaging system and calculated by comparing individual samples bands.
RESULTS
Kv4 family α- and β-subunit expression in the rat BLA
Although KChIP1 has been reported to be selectively expressed by GABAergic neurons in other forebrain structures, nothing is known about the relative expression of the β-subunits of the Kv4 potassium channel in neurons of the BLA. Hence, we first used immunofluorescence staining to determine the pattern of KChIPs protein expression in free-floating sections of the BLA.
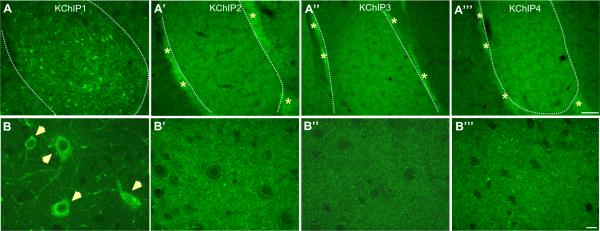
Our immunofluorescence studies revealed that all four β-subunits of the Kv4 potassium channel were present at high levels relative to surrounding tissue; however, only KChIP1 showed high somatodendritic immunoreactivity in neurons of the BLA (Fig. 1A, B). In contrast, although KChIP2 and KChIP4 were also found at high levels in the BLA (Fig. 1A′,A‴) their immunofluorescence was limited to the neuropil and to the pericapsular clusters of intercalated cell masses (ICM) (Fig. 1A, starred) surrounding the lateral and BLA complex. Moreover, KChIP2 and KChIP4 showed more punctate-like immunoreactivity in the BLA neuropil suggesting that these two subunits might be highly expressed in the spines of BLA principal neurons (Fig. 1B′,B‴). KChIP3 showed moderate immunoreactivity in the neuropil of the BLA as well as in the ICM (Fig. 1A″, B″).
Fig. 1.
Photomicrographs showing immunoreactivity and distribution of β-subunits of Kv4 potassium channel: KChIP1, 2, 3 and 4 in the rat BLA. (A–A‴) At the low magnification (10×, scale bar 100 μm) it can be seen that KChIP1 is the only β-subunit of Kv4 potassium channel which represents somatodendritic immunoreactivity in the rat BLA, while other KChIPs are mainly cumulated in the clusters of intercalated cell masses (ICM, as indicated by asterisks) as well as in the BLA neuropil. (B–B‴) As seen at higher magnification (63×, scale bar 10 μm) KChIP1 is predominantly expressed in cell somas and proximal dendrites thorough the BLA (B, closed arrows), while other KChIPs represent high neuropil immunoreactivity. Dashed lines represent the lateral boundaries of the BLA. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
We next examined the expression pattern for the Kv4 family of α-subunits to see if one or more of them had a similar expression pattern to KChIP1. All three Kv4 family α-subunits were expressed in the rat BLA and all showed high neuropil immunoreactivity (Fig. 2). However, the highest immunoreactivity was observed for Kv4.3, which was also the only α subunit observed clearly in the somatodendritic compartment of BLA neurons of diverse size and shape, with the highest density being observed in the basolateral nucleus (Fig. 2A″, B″).
Fig. 2.
Photomicrographs showing immunoreactivity of α-subunits of Kv4 potassium channel: Kv4.1, Kv4.2, and Kv4.3 in the rat BLA. (A–A″) All α-subunits of Kv4 potassium channel show high and distinct immunoreactivity in the rat BLA. All subunits are present in the BLA neuropil, but Kv4.2 is predominantly expressed in the ICM as indicated by asterisks (A′), while Kv4.3 represents the highest somatodendritic immunoreactivity comparing to other α-subunits (A″) as seen at low magnification (10×, scale bar 100 μm). (B–B″) Kv4.1 subunit is present mainly in the BLA neuropil as well as neurons” nuclei (B), while Kv4.2 subunit is highly expressed in the BLA neuropil but also processes throughout the BLA and cytoplasmatic/nuclear compartment of neurons (B′). Kv4.3 subunit is the only α-subunit of Kv4 potassium channel predominantly expressed in somas and dendrites of the BLA neurons (B″,63×, scale bar 10 μm). Dashed lines represent the lateral boundaries of the BLA. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
The highest Kv4.2 immunoreactivity was found in the ICM as well as the neuropil of the BLA (Fig. 2A′). Kv4.2 was also seen in soma of neurons scattered throughout the entire BLA (Fig. 2B′). Occasionally, some smaller sized cells (possibly neurogliaform cells) were observed that showed membrane-specific expression of this subunit (not shown). Finally, Kv4.1 was found at moderate levels throughout the BLA as strong punctate-like immunolabeling in the neuropil, as well as in the soma of neurons (Fig. 2A, B). The similarity in the staining patterns for KChIP1 and Kv4.3 raised the possibility that they may be co-localized in BLA neurons.
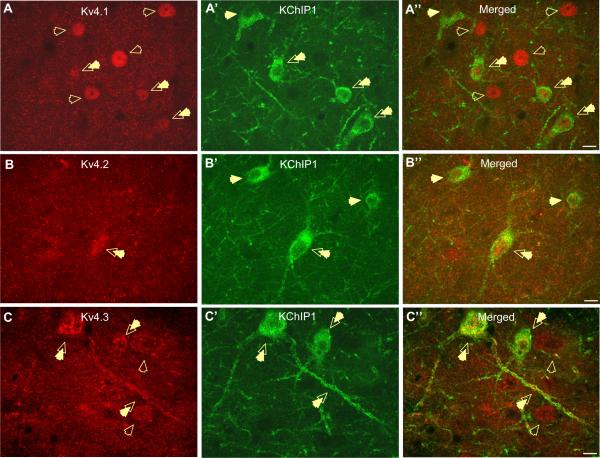
As illustrated in Fig. 3, all of the α-subunits were seen to co-localize with KChIP1. Hence, the majority of KChIP1-positive neurons express Kv4.1 and Kv4.2 (Fig. 3A–A″, B–B″). However, the signal for these α-subunits was only present in the soma of neurons co-localizing KChIP1, and not in neuronal membranes or dendrites (Fig. 3A–A″, B–B″). Overall, 67% of KChIP1-positive neurons express Kv4.1, whereas 48% of Kv4.1-positive cells express KChIP1 (Fig. 3A″). Similarly, 80% of KChIP1-positive neurons express Kv4.2, whereas 45% of Kv4.2-positive cells express KChIP1 (Fig. 3B″). Intriguingly, those Kv4.1 and Kv4.2-positive neurons, which do not express KChIP1, have more intense staining and have a larger cell soma than those, which do express KChIP1 (Fig. 3A″, B″). Finally, a small subpopulation of neurons was observed having only membrane-specific expression of Kv4.2 and these neurons did not co-express KChIP1 (not shown).
Fig. 3.
Photomicrographs showing co-expression of the auxiliary β subunit KChIP1 with the Kv4.1, Kv4.2, and Kv4.3 α-subunits of the A-type potassium channel in neurons of the rat BLA. (A–A″) The expression pattern of the Kv4.1 subunit (A; red) appears to be restricted to nuclear and cytoplasmatic compartment of neurons. The majority of Kv4.1-expressing neurons do not co-express KChIP1 as indicated by open arrows (A″, merged). However, a small population of neurons are observed to express Kv4.1-subunit in cytoplasm/nucleus and co-express KChIP1 in membrane and proximal dendrites as indicated by double arrows (A″; merged). (B–B″) Low level co-localization is observed for the Kv4.2 α-subunit and KChIP1 subunit in neurons of the BLA. Kv4.2 subunit shows high immunoreactivity in the BLA neuropil (B; red) Occasionally Kv4.2 is concentrated in the nucleus and cytoplasm of BLA neurons, and some of these neurons co-localize KChIP1 (B″, double arrow). (C–C″) Photomicrographs showing a high levels of co-localization of Kv4.3 α-subunit with KChIP1 in the soma and proximal dendrites of BLA neurons of diverse shape and size (C″, merged, double arrows). Occasionally, there are Kv4.3-positive neurons, which do not express KChIP1 β-subunit (C″, merged, open arrows). All photographs at 63× magnification, scale bar 10 μm. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Significantly, our dual-immunofluorescence studies revealed an almost complete overlap in the localization of KChIP1 with Kv4.3 in the somatodendritic compartment of a large number of neurons scattered throughout the BLA. Moreover, 95% of KChIP1-positive neurons were positive for Kv4.3, and 91% of Kv4.3-positive neurons were positive for KChIP1 (Fig. 3C–C″). These results are summarized in Table 2.
Table 2.
Table showing co-localization of (β-subunit KChIP1 with α subunits of potassium channel: Kv4.1, Kv4.2 and Kv4.3 with markers for defined subpopulations of BLA interneurons in the rat BLA
| % marker cells co-expressing KChIP1 | % KChIP1 cells expressing marker | |
|---|---|---|
| Kv4.1 (n=8) | 48% (82/170) | 67% (82/122) |
| Kv4.2 (n=7) | 45% (91/204) | 80% (91/114) |
| Kv4.3 (n=8) | 91% (92/101) | 95% (92/97) |
| Parvalbumin (n=8) | 94% (45/48) | 35% (45/127) |
| Somatostatin (n=7) | 65% (22/34) | 19% (22/114) |
| Cholecystokinin-8 (large, n=6) | 100% (10/10) | 10% (10/103) |
| Cholecystokinin-8 (small, n=6) | 6% (1/16) | 13% (16/121) |
| Calretinin (n=8) | 11% (4/36) | 3% (4/125) |
| Neuropeptide Y (n=7) | 39% (6/15) | 7% (6/89) |
n—total number of sections analyzed, average number of immunoreactive cells counted per section is indicated in brackets.
Western blot analysis and co-immunoprecipitation experiments
Although our data suggested that KChIP1 and the Kv4s showed a relatively high degree of co-localization in sub-populations of BLA neurons these studies could not determine if a functional association existed between the two proteins. Moreover, KChIP1 with Kv4.3 were observed in the same cellular compartments and showed>90% co-localization suggesting that these two subunits may combine in a functional macromolecule. To address this issue, we next conducted a Western blot and co-IP study to test for possible protein-protein interactions between the different subunits. Here, we first ran a Western blot assay to obtain an estimate of protein expression levels in the BLA. As shown in Fig. 4A all four KChIP β-subunits and all three Kv4 family α-subunits were detected as distinct bands in immunoblots. Consistent with previous reports (Rhodes et al., 2004), Kv4.2 and Kv4.3 were observed as single bands at 72 and 70 kDa, respectively. In contrast, Kv4.1 showed two bands, a strong band at 87 kDa and a weaker band at 60 kDa. The strongest Kv4 signal in the BLA was obtained for Kv4.3 and Kv4.1 (upper band) (integrated intensity value (IIV) as compared to GAPDH intensity; IIV=0.11± 0.04, and 0.08±0.03, respectively), and Kv4.2 had the weakest signal (IIV=0.04±0.03) (Fig. 4A). Among the KChIP subunits, the strongest signal was observed for KChIP2 (IIV=1.21±0.38), moderate signal was observed for KChIP4 (IIV=0.42±0.11), and the weakest signal was observed for KChIP3 and KChIP1 (IIV=0.12±0.02 and 0.08±0.04, respectively) (Fig. 4A).
We next used KChIP1 as bait protein in a co-IP assay to determine if KChIP1 forms a protein-protein association with the Kv4 α-subunits. A rabbit antibody raised against the Kv3.2 potassium channel was used as a negative control in this study. Surprisingly, not only was KChIP1 seen to co-immunoprecipitate with Kv4.3, but also with Kv4.1 and Kv4.2 as shown in Fig. 4B. As expected Kv3.2 failed to co-precipitate with KChIP1 in any BLA sample tested (Fig. 4B). However, in agreement with a previous immunohistochemical study (McDonald and Mascagni, 2006), BLA tissue showed a high expression of Kv3.2 protein as confirmed by Western blot (Fig. 4B).
KChIP1 expression in defined subpopulations of BLA neurons
Having determined that KChIP1 forms a macromolecular complex with Kv4s in what appeared to be a discrete subpopulation of BLA neurons, we next examined if this co-localization was also restricted to a defined subpopulation of interneurons or principal neurons. Because the somatodendritic expression pattern for KChIP1 was highly reminiscent of PV staining in the BLA (McDonald and Mascagni, 2001) we first examined the co-localization of KChIP1 with different neurochemical markers of interneuron subpopulations. Here we used dual-immunofluorescence to determine the relative co-expression of KChIP1 with PV, SOM, CCK-8, CR, and NPY.
As we detail below, KChIP1 is expressed in every major interneuron subpopulation, with the highest level of co-expression seen in the PV, CCK-8, and SOM subpopulations (Figs. 5 and 6, Table 2). Finally, to evaluate the relative expression of KChIP1 in principal neurons we used an antibody against calcium/calmodulin-dependent protein kinase II α (CaMKIIα), which is selectively expressed in these neurons (McDonald et al., 2002).
Fig. 5.
Photomicrographs showing a high level of co-localization of KChIP1-positive neurons with PARV, SOM and CCK-8-immunoreactive interneurons of the BLA. (A7#x2013;A″) PARV interneurons (A; red) are seen to co-localize KChIP1 (A′; green) in the soma and dendrites. KChIP1 expression was found in 94% of the PARV-expressing interneurons (A″; merged, double arrows); however, there are KChIP1-positive neurons, which do not co-express PARV (A″; merged, closed arrow). (B–B″) KChIP1 and SOM-immunoreactive neurons extensively co-localize at the level of neurons' membrane and cytoplasm (B″, merged, double arrows). (C–C″) Large (L-type) CCK-8 interneurons characterized by large somata and thick dendrites co-localize with KChIP1 β-subunit at the level of membrane/cytoplasm and proximal dendrites in the BLA (C″; merged, double arrows). All photographs at 63× magnification, scale bar 10 μm. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Fig. 6.
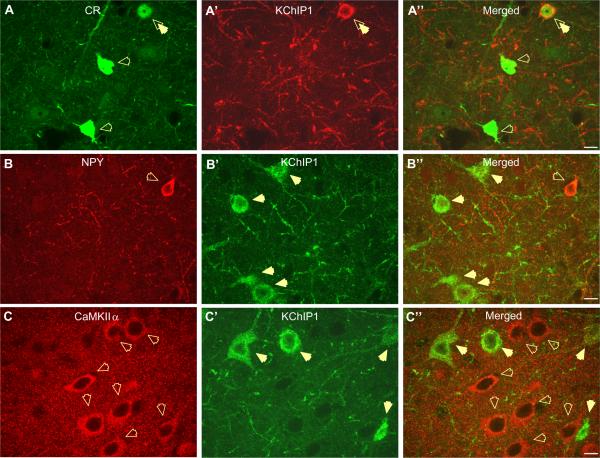
Photomicrographs showing low level of co-localization of KChIP1-positive neurons with the CR, NPY and CaMKII α. (A–A″) The great majority of CR-expressing neurons (A; green) do not co-localize with KChIP1-immunoreactive neurons in the BLA (A″; merged). Open arrows indicate CR-expressing interneurons, which do not co-express KChIP1 and double arrow indicates neuron co-localizing CR and KChIP1 (A″; merged). (B–B″) Photomicrographs showing low level co-localization of KChIP1-positive neurons with NPY subpopulation of the BLA interneurons. As indicated by open arrow, NPY-positive interneuron does not co-express KChIP1 (B″, merged). Closed arrows show KChIP1-positive neurons, which do not co-express NPY (B′, green). (C–C″) Photomicrographs showing exclusive immunoreactivity pattern of Calcium/Calmodulin-dependent protein kinase A alpha (CaMKII α) and KChIP1 in the rat BLA. All CaMKII α-immunoreactive neurons (C; red, open arrows) are seen to be exclusively localized with KChIP1-positive neurons (C′; green, closed arrows). All photographs at 63 magnification, scale bar 10 μm. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
As expected, KChIP1 was found to co-localize in almost every PV-immunoreactive interneuron in both lateral amygdala (LA) and BLA. Thus, KChIP1 was detected in 94% of PV-positive interneurons, whereas 65% of KChIP1-positive neurons do not express PV (Fig. 5A–A″). However, KChIP1 was not found in perisomatic baskets formed by PV-positive neurons (Fig. 5A″). We next examined the relative co-expression of KChIP1 with the remaining markers for BLA interneurons.
Like PV-positive interneurons, the majority of SOM-immunoreactive interneurons also co-express KChIP1. However, a subpopulation of classic fusiform-shaped SOM interneurons was observed that do not express KChIP1. Nonetheless, 64% of the SOM-immunoreactive interneurons co-expressed KChIP1 (Fig. 5B–B″), and 19% of KChIP1-positive cells are SOM-positive.
As illustrated in Fig. 5C–C″, the largest KChIP1-positive neurons were also immunoreactive for CCK-8. These neurons are similar to those previously termed large (L-type) CCK interneurons (Mascagni and McDonald, 2003), and characteristically had a large soma and thick dendrites, compared to other CCK interneurons. All large CCK interneurons in the BLA co-expressed KChIP1, whereas only 10% of KChIP1 cells were CCK-positive. In contrast, only 6% of the remaining CCK interneurons, termed small (S-type) interneurons, co-expressed KChIP1 (Fig. 5C–C″).
The majority (88%) of CR-positive interneurons do not co-localize KChIP1 (Fig. 6A–A″), and CR-positive neurons represent only 3% of the KChIP1-positive cell population. Moreover, those CR interneurons that do co-express KChIP1 are regionally distributed. Thus, no co-localization was ever observed in the rostral BLA, whereas in the caudal BLA classic bipolar CR neurons were seen to co-express KChIP1. Like CR-positive interneurons, only 39% of the NPY-positive interneurons co-expressed KChIP1 (Fig. 6B–B″), and these neurons represent only 7% of the KChIP1-positive cell population.
Unlike BLA interneurons, principal neurons of the BLA do not express KChIP1. As illustrated in Fig. 6C, KChIP1 immunoreactivity and CaMKIIα immunoreactivity are mutually exclusive such that no KChIP1-positive neurons were seen to express CaMKIIα (Fig. 6C–C″). These data strongly support the selective expression of KChIP1 in discrete subpopulations of BLA interneurons.
DISCUSSION
Our studies clearly demonstrate that KChIP1, an auxiliary β-subunit of Kv4 potassium channels, is exclusively expressed in the somatodendritic compartment of PV-, SOM-, and large CCK-immunoreactive interneurons in the BLA. Although KChIP2–4 were also found in the BLA, their expression was restricted to the neuropil, as well as the ICM, a pattern that makes it difficult to determine cell type specificity for these KChIPs using light microscopy. Similarly, all three Kv4 α-subunits are found in the rat BLA and show a considerable overlap with KChIP1 immunofluorescence, ranging from 67 to 91%. Moreover, KChIP1 and Kv4 α-subunits co-immunoprecipitate, further suggest that KChIP1 could functionally regulate the activity of A-type potassium channels in BLA interneurons containing the Kv4 α subunits. By extrapolation, therefore, macromolecular complexes containing KChIP1 and the Kv4s are predicted to be found at high levels in the somatodendritic compartment of PV-, SOM-, and large CCK-immunoreactive BLA interneurons. Given the key role played by BLA inhibitory circuits in fear memory formation and extinction, modulation of KChIP1 expression in the BLA may represent a novel target for regulating affective processes.
Although all four KChIPs are found in the amygdala, there are clear differences in their regional, cellular, and subcellular localization. For example, all four KChIPs were expressed at higher levels in the basolateral complex than in the surrounding tissue, and even within the basolateral complex only KChIP1 showed a clear somatodendritic localization, whereas KChIP2–4 were observed predominantly in the neuropil. Similarly, KChIP2–4 were found at high levels in the pericapsular ICM, in contrast to KChIP1 which was never observed in the ICM. Finally, the pattern of KChIP1 staining suggested that this β subunit was preferentially expressed by a population of GABAergic BLA interneurons. Because of the apparent differences in compartmentalization for the KChIPs, it is possible that KChIP2–4 are found predominantly in principal neurons and not in BLA interneurons, as has been suggested by in situ hybridization studies (Serodio and Rudy, 1998) and immunohistochemical studies (Rhodes et al., 2004). However, the ICM also show high KChIP2–4 expression and these are a heterogeneous population of small GABAergic neurons (Millhouse, 1986; Pare and Smith, 1993). Hence, while KChIP1 does appear to localize selectively to GABAergic interneurons in the BLA, it should not be considered as a unique identifier of GABAergic neurons in the amygdala as a whole.
Like the KChIPs, the distribution of the Kv4 α subunits in the BLA also showed both regional and subcellular differences. Kv4.1 demonstrated strong punctate-like immunolabeling in the neuropil, which suggests the presence of Kv4.1 subunit in spines and processes throughout the BLA. However, apparent Kv4.1 immunoreactivity was also observed in the soma/nucleus of BLA neurons, which is not a typical pattern of immunolabeling expected for a voltage-gated potassium channel. While the Kv4.1 antibody has passed all available tests for specificity, in the absence of Kv4.1 knockout mice, we cannot rule out the possibility that the soma/nuclear staining is non-specific. Nonetheless, while 48% of Kv4.1 neurons co-expressed KChIP1, an equal number (52%) did not co-localize, suggesting that the Kv4.1 subunit is also found in BLA principal neurons. Although we have shown relatively high expression of Kv4.1 in the BLA, Northern blot analysis and in situ hybridization studies have shown that Kv4.1 transcripts are only present at very low levels in the CNS when compared to Kv4.2 and Kv4.3 (Serodio and Rudy, 1998). However, these authors suggested that the Kv4.1 transcript might be translated in higher efficiency, and/or have a longer lifetime in the cytoplasm, which might explain the apparent discrepancy.
Although Kv4.2 immunoreactivity was generally observed in the BLA neuropil, we also observed a subpopulation of sparsely distributed smaller neurons, in which Kv4.2 expression was restricted to the cell membrane. However, because of the strong neuropil expression of Kv4.2, the total number of Kv4.2-positive neurons might be higher than observed. At present the identity of this sub-population of neurons remains unknown.
Interestingly, while all three Kv4s were observed in the basolateral complex, only Kv4.2 was found in the ICM, suggesting that in this cell population the KChIPs would regulate homomeric Kv4.2 channels. It is possible that more than one KChIP may be found in ICM neurons and, hence, control of Kv4.2 channel function in the ICM may be more complex than that occurring in BLA interneurons. Interestingly, high levels of μ opioid receptors and dopamine D1 receptors are found in the IMC of both rats and primates (Fuxe et al., 2003; Muly et al., 2009), and it is possible that one or both of these transmitter systems regulate the firing activity of GABAergic ICM neurons by modulating the activity of Kv4.2 channels.
Finally, Kv4.3 was the only subunit to show a clear somatodendritic expression pattern, which was remarkably similar to that of KChIP1 expression. Consistent with our findings, cell type-specific Kv4.3 expression has been reported for large multipolar interneurons of the dentate gyrus and hippocampal CA3 region, medium to large multipolar striatal interneurons, as well as in neocortical interneurons of all morphological classes (Rhodes et al., 2004). In contrast, in the hippocampus and neocortex Kv4.2 subunit is mainly expressed in the apical and basal dendrites of glutamatergic pyramidal neurons, where it is found in association with KChIP2, 3, and 4 (Rhodes et al., 2004).
Our assertion that KChIP1 may be preferentially expressed by BLA interneurons was further confirmed by our dual-immunofluorescence study. Here, KChIP1 was found to co-express in every BLA interneuron subpopulation, where it was found at highest levels in PV-positive (94%), SOM-positive (65%), and large CCK interneurons (100%), but not in CaMKIIα-positive principal neurons. Additionally, we haven't observed KChIP1 expression inside the perisomatic baskets formed by PV interneurons (Fig. 5A″). Since PV neurons, in contrast to other interneurons, are known to innervate mainly BLA projection neurons (Muller et al., 2003), this observation confirms preferential KChIP1 expression in the BLA interneurons. Co-expression in NPY-positive interneurons was moderate (39%), while only 11% of CR-positive interneurons expressed KChIP1. Consistent with our results, 60% of hippocampal PV interneurons are positive for KChIP1 (Menegola et al., 2008), and all PV-positive neurons in the cerebellum and the reticular thalamus are reported to express KChIP1 (Xiong et al., 2009). In contrast, PV interneurons of the medial habenullar nuclei do not express KChIP1 (Xiong et al., 2009). Hence, KChIP1 expression is not necessarily synonymous with PV expression.
Interestingly, while there is no overlap in the expression of PV, CCK, and SOM in BLA interneurons, each of these subpopulations also co-express CB (McDonald, 1994; McDonald and Mascagni, 2002). Hence, expression of KChIP1 may positively correlate with CB expression rather than with more specific interneuron markers. Consistent with this hypothesis, one-third of SOM interneurons in the BLA also express NPY (McDonald and Mascagni, 2002; Levita et al., 2003), and ~40% of NPY-positive interneurons express KChIP1, hence these neurons may represent a single population of CB-, SOM-, and NPY-positive interneurons. In contrast, the majority of calretinin and small CCK interneurons do not express KChIP1. However, these markers are known to overlap with each other (Mascagni and McDonald, 2003), but CR is mutually exclusive with CB (McDonald and Mascagni, 2002). Thus, the apparent heterogeneity of KChIP1 expressing BLA interneurons might in fact subdivide into two main groups: CB-like interneurons expressing KChIP1 (PV, large CCK, SOM, and some NPY), and calretinin-like interneurons that do not co-express KChIP1 (CR, small CCK-8, and the majority of NPY).
Consistent with our immunofluorescence studies, KChIP2 and KChIP4 showed the strongest signal in our Western blots, whereas the KChIP3 signal was moderate, and KChIP1 showed the weakest signal. Since principal neurons constitute ~80% of the BLA neuronal population, hence the relatively high expression of KChIP2 and KChIP4 might reflect preferential expression in principal neurons, and a weak signal for KChIP1 would be predicted if, as we propose, it is preferentially expressed in BLA interneurons.
Significantly, our co-IP study revealed that all three Kv4 α-subunits co-precipitated with KChIP1 in the BLA. This was a little surprising given that the strongest signal for Kv4.1 and Kv4.2 immunoreactivity was observed in apparently different compartments of BLA interneurons. However, Kv4.1 and Kv4.2 immunoreactivity had a punctuate appearance in the BLA neuropil and it is possible that KChIP1 is co-expressed with these subunits at highly localized specializations in BLA interneurons. Indeed, clusters of Kv4.3 channels have been reported to form specialized junctions between climbing fiber terminals and the somatodendritic surface of cerebellar interneurons (Kollo et al., 2006). We are conducting a high resolution electron microscopic study to determine if Kv4.1 and Kv4.2 form similar clusters in BLA interneurons. Moreover, Kv4 α subunits can form heteromeric as well as homomeric channels (Wang et al., 1993). Hence, in BLA interneurons KChIP1 may regulate the activity of A-type channels containing more than one Kv4 α subunits. Consistent with this premise, a previous study has reported a co-association between Kv4.2 and Kv4.3 in the co-IP experiment (Rhodes et al., 2004), which might help to explain the co-existence of all three Kv4 subunits in some BLA interneurons. Nevertheless, our immunofluorescence would strongly suggest that KChIP1 and Kv4.3 are preferential binding partners, and a similar preference has been reported for interneurons in the hippocampus, neocortex, and striatum (Rhodes et al., 2004). However, we cannot discount the possibility that KChIP1 may preferentially bind to different Kv4 partners, depending on the interneuron subtype and contribute to their unique physiological properties.
BLA interneurons can be differentiated into at least four electrophysiologically distinct subtypes according to their characteristic firing pattern: namely the burst-firing (BF), regular-firing (RF), fast-firing (FF), and stutter-firing (SF) interneurons. Over 60% of BF and SF interneurons express PV (Rainnie et al., 2006), and similar subpopulations of interneurons have been identified in the frontal cortex, sensory-motor cortex, hippocampus and neostriatum (Kawaguchi and Kubota, 1996; Kawaguchi and Kondo, 2002; Markram et al., 2004). Significantly, although there is evidence that the electrophysiological properties of interneurons are dependent on the activation of multiple ion channels (Rudy and McBain, 2001; Toledo-Rodriguez et al., 2004), a recent cluster analysis has revealed that the firing properties of neocortical interneurons can be best predicted by their PV-, CB-, or CR-immunoreactivity (Toledo-Rodriguez et al., 2004). Since PV- and CR-positive interneurons of the BLA show markedly different expression patterns for KChIP1, this raises the question of how the functional interaction between KChIP1 and the Kv4s may help determine the electrophysiological properties of discrete interneuron subtypes.
Each KChIP1 subunit has been shown to tether two Kv4.3 subunits, and hence strongly regulate the surface expression, and subunit assembly of Kv4 channels (Cui et al., 2008; Wang, 2008). Moreover, Lacaille and colleagues have shown that a subset of hippocampal GABAergic interneurons display a prominent IA current that is mediated by activation of Kv4.3 channels (Bourdeau et al., 2007). Intriguingly, Nerbonne and colleagues (Burkhalter et al., 2006) have shown that clusters of Kv4.2 and Kv4.3 subunits are excluded from excitatory synapses, but included in the GABAergic synapses of interneurons in the mouse primary visual cortex. McDonald and coworkers have shown that while PV-positive interneurons preferentially innervate principal neurons, CR-positive interneurons preferentially innervate other interneurons (Muller et al., 2003). Hence, co-expression of KChIP1 and Kv4.3 may selectively function to regulate inhibitory synaptic input from CR-positive interneurons onto PV-, SOM-, and large CCK interneurons. Parvalbumin interneurons of the BLA make multiple perisomatic contacts with principal neurons and thus provide strong inhibitory control over principal neuron excitability (McDonald and Mascagni, 2001; Rainnie et al., 2006). As noted above, the KChIP1 also belongs to a family of neuronal calcium sensor proteins (NCS) that bind Ca2+ and, in doing so, induce a conformational change in their structure and function (O'Callaghan and Burgoyne, 2003; O'Callaghan et al., 2003, Sours-Brothers et al., 2009). Hence, KChIPs might respond to alterations of intracellular calcium, and relay activity-dependent information to a key subpopulation of BLA interneurons. Recently, Anderson and colleagues have shown that binding of Ca2+ to KChIP3 caused a leftward shift in the activation threshold for Kv4 channels in stellate cells of the cerebellum, thereby regulating neuronal firing activity (Anderson et al., 2010). If KChIP1 causes a similar leftward shift in the activation threshold of Kv4.3 in PV-positive interneurons of the BLA, modulation of KChIP1 function could have a significant impact on inhibitory control of principal neurons. Consistent with this hypothesis, KChIP1-knockout mice show an increased susceptibility to pentylenotetrazole-induced seizures (Xiong et al., 2009), and the induction of status epilepticus increases the expression KChIP1 and Kv4.3 mRNA and proteins in amygdala (Chang et al., 2006), and hippocampus (Su et al., 2008). Together these data suggest that the expression of KChIP1 and Kv4.3 in BLA interneurons is activity-dependent, and may be grossly disrupted by environmental factors that are known to precipitate seizure activity, such as chronic stress.
CONCLUSION
In conclusion, we have shown that all three α-subunits and all four β-subunits of Kv4 potassium channel are present in the neurons of the rat BLA, but only KChIP1 and Kv4.3 subunit appear to be preferentially expressed in a distinct subpopulation of interneurons. Furthermore KChIP1 appears to be the major determinant in regulating the somatodendritic A-current in calbindin-like BLA interneurons (e.g. the PV, large CCK, and SOM-expressing interneurons). Our results confirm previous findings from other brain regions and suggest that KChIP1 might be a specific modulator of the GABAergic inhibitory network in the BLA.
Acknowledgments
We would like to thank Dr. Chris Muly for his invaluable input in the preparation of this manuscript. This work was supported by National Institute of Mental Health Grant MH069852 to D.G. Rainnie; the Science and Technology Centers Integrative Partnership Program of the National Science Foundation (The Center for Behavioral Neuroscience) Grant IBN-987675; and the Yerkes National Primate Research Center Base Grant RR-00165 awarded by the Animal Resources Program of National Institutes of Health.
Abbreviations
- BCA
bicinchoninic acid
- BLA
basolateral amygdala
- CaMKIIα
calcium/calmodulin-dependent protein kinase II α
- CB
calbindin
- CCK-8
cholecystokinin 8
- co-IP
co-immunoprecipitation
- CR
calretinin
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- ICM
pericapsular clusters of intercalated cell masses
- KChIP1–4
potassium channel interacting protein 1–4
- Kv3.2
potassium channel Kv3.2
- Kv4.1–Kv4.3
alpha subunit of Kv4.1, Kv4.2, and Kv4.3 potassium channel
- NPY
neuropeptide Y
- PBS
phosphate buffer saline
- PV
parvalbumin
- SOM
somatostatin
REFERENCES
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- Anderson D, Mehaffey WH, Iftinca M, Rehak R, Engbers JD, Hameed S, Zamponi GW, Turner RW. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci. 2010;13:333–337. doi: 10.1038/nn.2493. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–833. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- Bourdeau ML, Morin F, Laurent CE, Azzi M, Lacaille JC. Kv4.3-mediated A-type K+ currents underlie rhythmic activity in hippocampal interneurons. J Neurosci. 2007;27:1942–1953. doi: 10.1523/JNEUROSCI.3208-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter A, Gonchar Y, Mellor RL, Nerbonne JM. Differential expression of I(A) channel subunits Kv4.2 and Kv4.3 in mouse visual cortical neurons and synapses. J Neurosci. 2006;26:12274–12282. doi: 10.1523/JNEUROSCI.2599-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, Su T, Sun WW, Zhao QH, Qin B, Liao WP. ([Changes of potassium channels Kv4.2, Kv4.3 and Kv channel interacting protein 1 in amygdala kindling epilepsy: experiment with rats] Zhonghua Yi Xue Za Zhi. 2006;86:3315–3318. [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Covarrubias M, Bhattacharji A, De Santiago-Castillo JA, Dougherty K, Kaulin YA, Na-Phuket TR, Wang G. The neuronal Kv4 channel complex. Neurochem Res. 2008;33:1558–1567. doi: 10.1007/s11064-008-9650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YY, Liang P, Wang KW. Enhanced trafficking of tetrameric Kv4.3 channels by KChIP1 clamping. Neurochem Res. 2008;33:2078–2084. doi: 10.1007/s11064-008-9688-7. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Jacobsen KX, Hoistad M, Tinner B, Jansson A, Staines WA, Agnati LF. The dopamine D1 receptor-rich main and paracapsular intercalated nerve cell groups of the rat amygdala: relationship to the dopamine innervation. Neuroscience. 2003;119:733–746. doi: 10.1016/s0306-4522(03)00148-9. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Ressler KJ, Hammack SE, Chhatwal JP, Rainnie DG. Distinct subtypes of cholecystokinin (CCK)-containing interneurons of the basolateral amygdala identified using a CCK promoter-specific lentivirus. J Neurophysiol. 2009;101:1494–1506. doi: 10.1152/jn.91149.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31:277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci. 1996;16:2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Davis M. Electrolytic lesions of the amygdala block acquisition and expression of fear-potentiated startle even with extensive training but do not prevent reacquisition. Behav Neurosci. 1993;107:580–595. doi: 10.1037//0735-7044.107.4.580. [DOI] [PubMed] [Google Scholar]
- Kollo M, Holderith NB, Nusser Z. Novel subcellular distribution pattern of A-type K+ channels on neuronal surface. J Neurosci. 2006;26:2684–2691. doi: 10.1523/JNEUROSCI.5257-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- Levita L, Mania I, Rainnie DG. Subtypes of substance P receptor immunoreactive interneurons in the rat basolateral amygdala. Brain Res. 2003;981:41–51. doi: 10.1016/s0006-8993(03)02870-1. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976:171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Calretinin immunoreactive neurons in the baso-lateral amygdala of the rat and monkey. Brain Res. 1994;667:238–242. doi: 10.1016/0006-8993(94)91501-6. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 2001;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Differential expression of Kv3.1b and Kv3.2 potassium channel subunits in interneurons of the basolateral amygdala. Neuroscience. 2006;138:537–547. doi: 10.1016/j.neuroscience.2005.11.047. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol. 2002;446:199–218. doi: 10.1002/cne.10204. [DOI] [PubMed] [Google Scholar]
- Menegola M, Misonou H, Vacher H, Trimmer JS. Dendritic A-type potassium channel subunit expression in CA1 hippocampal interneurons. Neuroscience. 2008;154:953–964. doi: 10.1016/j.neuroscience.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegola M, Trimmer JS. Unanticipated region- and cell-specific downregulation of individual KChIP auxiliary subunit isotypes in Kv4.2 knock-out mouse brain. J Neurosci. 2006;26:12137–12142. doi: 10.1523/JNEUROSCI.2783-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol. 1986;247:246–271. doi: 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol. 2003;456:217–236. doi: 10.1002/cne.10435. [DOI] [PubMed] [Google Scholar]
- Muly EC, Senyuz M, Khan ZU, Guo JD, Hazra R, Rainnie DG. Distribution of D1 and D5 dopamine receptors in the primate and rat basolateral amygdala. Brain Struct Funct. 2009;213:375–393. doi: 10.1007/s00429-009-0214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan DW, Burgoyne RD. Role of myristoylation in the intracellular targeting of neuronal calcium sensor (NCS) proteins. Biochem Soc Trans. 2003;31:963–965. doi: 10.1042/bst0310963. [DOI] [PubMed] [Google Scholar]
- O'Callaghan DW, Hasdemir B, Leighton M, Burgoyne RD. Residues within the myristoylation motif determine intracellular targeting of the neuronal Ca2+ sensor protein KChIP1 to post-ER transport vesicles and traffic of Kv4 K+ channels. J Cell Sci. 2003;116:4833–4845. doi: 10.1242/jcs.00803. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57:1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Mania I, Mascagni F, McDonald AJ. Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. J Comp Neurol. 2006;498:142–161. doi: 10.1002/cne.21049. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, An WF, Trimmer JS. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–7915. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Sah P, Westbrook RF. Behavioural neuroscience: the circuit of fear. Nature. 2008;454:589–590. doi: 10.1038/454589a. [DOI] [PubMed] [Google Scholar]
- Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Doyère V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Sours-Brothers S, Ma R, Koulen P. Ca2+-sensitive transcriptional regulation: direct DNA interaction by DREAM. Front Biosci. 2009;14:1851–1856. doi: 10.2741/3346. [DOI] [PubMed] [Google Scholar]
- Su T, Cong WD, Long YS, Luo AH, Sun WW, Deng WY, Liao WP. Altered expression of voltage-gated potassium channel 4.2 and voltage-gated potassium channel 4-interacting protein, and changes in intracellular calcium levels following lithium-pilocarpine-induced status epilepticus. Neuroscience. 2008;157:566–576. doi: 10.1016/j.neuroscience.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Blumenfeld B, Wu C, Luo J, Attali B, Goodman P, Markram H. Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cereb Cortex. 2004;14:1310–1327. doi: 10.1093/cercor/bhh092. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wang K. Modulation by clamping: Kv4 and KChIP interactions. Neurochem Res. 2008;33:1964–1969. doi: 10.1007/s11064-008-9705-x. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive inter-neurons in the basolateral amygdala. J Neurosci. 2007;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Xia K, Li B, Zhao G, Zhang Z. KChIP1: a potential modulator to GABAergic system. Acta Biochim Biophys Sin (Shanghai) 2009;41:295–300. doi: 10.1093/abbs/gmp013. [DOI] [PubMed] [Google Scholar]